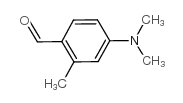
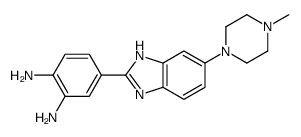
188247-01-0
| Name | N,N,3-trimethyl-4-[6-[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]-1H-benzimidazol-2-yl]aniline |
|---|---|
| Synonyms |
Methylproamine
CS-1306 |
| Description | Methylproamine is a DNA-binding radioprotector which, on the basis of published pulse radiolysis studies, acts by repair of transient radiation-induced oxidative species on DNA.IC50 Value: N/ATarget: DNA-binding radioprotectorin vitro: The extent of radioprotection at the clonogenic survival endpoint increased with methylproamine concentration up to a maximum dose modification factor (DMF) of 2.0 at 10 μM. At least 0.1 fmole/nucleus of methylproamine is required to achieve a substantial level of radioprotection (DMF of 1.3) with maximum protection (DMF of 2.0) achieved at 0.23 fmole/nucleus. The γH2AX focus yield per cell nucleus 45 min after irradiation decreased with drug concentration with a DMF of 2.5 at 10 μM [1]. Methylproamine-treated cells had fewer γH2AX foci after IR compared to untreated cells. Also, the presence ofmethylproamine decreased the amount of lower molecular weight DNA entering the gel as shown by the pulsed field gel electrophoresis assay [2]. Experiments with V79 cells have shown that methylproamine is approximately 100-fold more potent than the classical aminothiol radioprotector WR1065. The crystal structures of methylproamine and proamine complexes with the dodecamer d(CGCGAATTCGCG)(2) confirm that the new analogues also are minor groove binders [3].in vivo: N/AClinical trial: N/A |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C28H31N7 |
|---|---|
| Molecular Weight | 465.59300 |
| Exact Mass | 465.26400 |
| PSA | 67.08000 |
| LogP | 4.90230 |
| Storage condition | -20℃ |
|
~81% 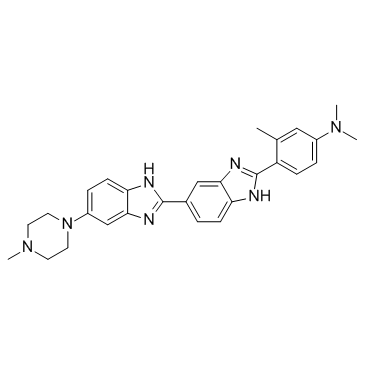
188247-01-0 |
| Literature: Peter MacCallum Cancer Institute Patent: EP2044938 A2, 2009 ; Location in patent: Page/Page column 21 ; |