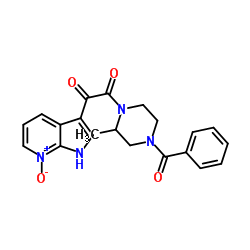
357263-13-9
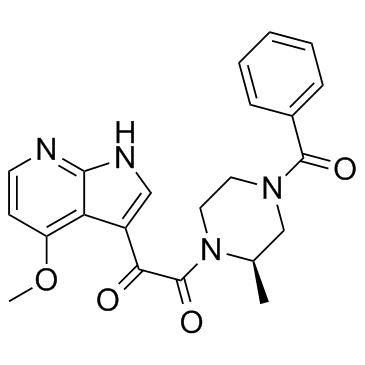
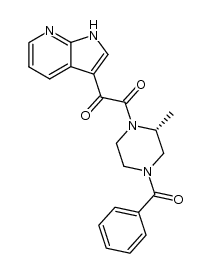
| Name | 1-[(2R)-4-benzoyl-2-methylpiperazin-1-yl]-2-(4-methoxy-1H-pyrrolo[2,3-b]pyridin-3-yl)ethane-1,2-dione |
|---|---|
| Synonyms |
1-[(2R)-4-Benzoyl-2-methyl-1-piperazinyl]-2-(4-methoxy-1H-pyrrolo[2,3-b]pyridin-3-yl)-1,2-ethanedione
1,2-ethanedione, 1-[(2R)-4-benzoyl-2-methyl-1-piperazinyl]-2-(4-methoxy-7H-pyrrolo[2,3-b]pyridin-3-yl)- (R)-N-(benzoyl)-3-methyl-N'-[(4-methoxy-7-azaindol-3-yl)-oxoacetyl]-piperazine 4-benzoyl-1-[(4-methoxy-1H-pyrrolo[2,3-b]pyridin-3-yl)oxoacetyl]-2-(R)-methylpiperazine Bms 806 1-[(2R)-4-benzoyl-2-methylpiperazin-1-yl]-2-(4-methoxy-7H-pyrrolo[2,3-b]pyridin-3-yl)ethane-1,2-dione Piperazine,4-benzoyl-1-[2-(4-methoxy-1H-pyrrolo[2,3-b]pyridin-3-yl)-1,2-dioxoethyl]-2-methyl-,(2R) 1,2-Ethanedione, 1-[(2R)-4-benzoyl-2-methyl-1-piperazinyl]-2-(4-methoxy-1H-pyrrolo[2,3-b]pyridin-3-yl)- (R)-1-(4-benzoyl-2-methylpiperazin-1-yl)-2-(4-methoxy-1H-pyrrolo[2,3-b]pyridin-3-yl)ethane-1,2-dione SINOVA SL-02580 BMS-378806 |
| Description | BMS-378806 is a potent HIV-1 attachment inhibitor that interferes with CD4-gp120 interactions. BMS-378806 selectively inhibits the binding of HIV-1 gp120 to the CD4 receptor with EC50 of 0.85-26.5 nM in virus. |
|---|---|
| Related Catalog | |
| Target |
HIV[1] |
| In Vitro | In a series of biochemical assays, BMS-378806 is not an effective inhibitor of HIV integrase, protease, or reverse transcriptase, but did compete with soluble CD4 binding to a monomeric form of gp120 in an ELISA assay with IC50=100 nM. The specificity of BMS-378806 toward inhibition of HIV-1 is confirmed by evaluation against HIV-2, SIV, MuLV, RSV, HCMV, BVDV, VSV, and influenza virus, with no significant inhibitory activity observed at concentrations ranging from 10 to 30 μM and no overt cytotoxicity toward the host cells, CC50>225 μM. BMS-378806 is not a potent inhibitor of any of the five major human CYP isoforms, evaluated as recombinant preparations, with IC50 values of >100 μM for CYP1A2 and CYP2C9, 23 μM for CYP2C19, 20 μM for CYP2D6, and 39 to 81 μM for CYP3A4. Moreover, since BMS-378806 is metabolized by CYP450 1A2, 2D6, and 3A4, it is unlikely to lead to severe drug−drug interactions in a clinical setting[1]. BMS-378806 inhibits viral replication by interfering with the binding interactions of gp120 with the cellular CD4 receptor. The IC50s determined for the gp120s from HIV LAI, BAL, NA420LN40, SF162, NL4-3, NA420B33, YU2, AD8, JRCSF, and 92US15.6 are 0.1, 0.1, 0.3, 0.5, 0.6, 0.7, 0.9, 1.0, 1.1, and 1.6 μM, respectively. A similar observation is also made for BMS-378806 (IC50s range from 0.2 to 9.6 μM)[2]. BMS-378806 binds directly to gp120 at a stoichiometry of approximately 1:1, with a binding affinity similar to that of soluble CD4. The potential BMS-378806 target site is localized to a specific region within the CD4 binding pocket of gp120 by using HIV-1 gp120 variants carrying either compound-selected resistant substitutions or gp120-CD4 contact site mutations[3]. |
| In Vivo | In toxicology studies, BMS-378806 is well tolerated in rats at doses of 100 mg/kg/day for 2 weeks and in dogs at doses of 90 mg/kg for 10 days. The dose-proportional increases in the AUC and Cmax are observed between doses of 5 and 25 mpk, when BMS-378806 is administered either in the solution or suspension formulation. In all three species, plasma levels of drug exceeded the concentrations required to half-maximally inhibit virus replication in vitro. The volume of distribution of BMS-378806 ranges from 0.4 to 0.6 L/kg, indicative of partitioning beyond plasma; however, examination of brain levels in the rat reveals minimal CNS penetration[1]. |
| Kinase Assay | To measure gp120-CD4 binding, the wild-type or variant gp120 proteins are first captured onto a plate by D7324 antibody. CD4 binding is initiated by adding sCD4 to a gp120-coated plate. To determine the ability of BMS-378806 to compete with sCD4 for gp120 binding, the compound is added simultaneously with sCD4 and reactions are carried out in buffer C (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1% bovine serum albumin) for 2 h at room temperature. After washing with buffer B (20 mM Tris-HCl, 500 mM NaCl, 0.05% Tween 20 [pH 7.5]), the bound CD4 is detected with OKT4 antibody (0.36 μg/mL) and goat anti-mouse peroxidase conjugate. Bound antibody is detected with 3,3′,5,5′-tetramethylbenzidine chromogenic substrate for peroxidase[3]. |
| Animal Admin | Rats, Dogs and Monkeys[1] The pharmacokinetic properties of BMS-378806 in the rat, dog, and cynomolgus monkey are summarized. The oral bioavailability of BMS-378806 in rats, administered as a solution in PEG 400/EtOH (90:10 v/v), is 19% at a dose of 5 mg/kg while an aqueous crystalline suspension of free base in 0.75% (w/w) Methocel A4M Premium administered orally at the same dose afforded a relative bioavailability of 61%. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 622.3ºC at 760 mmHg |
| Molecular Formula | C22H22N4O4 |
| Molecular Weight | 406.435 |
| Flash Point | 330.2ºC |
| Exact Mass | 406.164093 |
| PSA | 95.60000 |
| LogP | 0.73 |
| Index of Refraction | 1.647 |
| Storage condition | -20℃ |
| Precursor 9 | |
|---|---|
| DownStream 0 | |