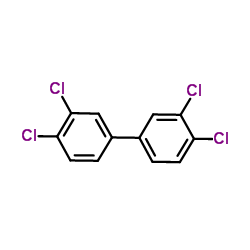
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
DV8650000
-
CHEMICAL NAME :
-
Biphenyl, 3,3',4,4'-tetrachloro-
-
CAS REGISTRY NUMBER :
-
32598-13-3
-
LAST UPDATED :
-
199710
-
DATA ITEMS CITED :
-
22
-
MOLECULAR FORMULA :
-
C12-H6-Cl4
-
MOLECULAR WEIGHT :
-
291.98
-
WISWESSER LINE NOTATION :
-
GR BG DR CG DG
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
1 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
6825 ug/kg/13W-C
-
TOXIC EFFECTS :
-
Liver - other changes Kidney, Ureter, Bladder - changes in bladder weight Endocrine - changes in spleen weight
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
150 mg/kg/3D-I
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - hepatic microsomal mixed oxidase (dealkylation, hydroxylation, etc.) Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - other Enzymes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
320 mg/kg/6D-I
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Endocrine - changes in thymus weight Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
108 mg/kg/18W-I
-
TOXIC EFFECTS :
-
Endocrine - evidence of thyroid hypofunction Endocrine - other changes Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Mammal - species unspecified
-
DOSE/DURATION :
-
150 mg/kg/3D-I
-
TOXIC EFFECTS :
-
Liver - other changes Nutritional and Gross Metabolic - weight loss or decreased weight gain Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - hepatic microsomal mixed oxidase (dealkylation, hydroxylation, etc.)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
39 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - stillbirth Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
39 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
110 mg/kg
-
SEX/DURATION :
-
female 8-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
39 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
13 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
224 mg/kg
-
SEX/DURATION :
-
female 10-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - biochemical and metabolic Reproductive - Effects on Newborn - behavioral
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
224 mg/kg
-
SEX/DURATION :
-
female 10-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
7 mg/kg
-
SEX/DURATION :
-
female 10-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
25 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
16 mg/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
630 ug/kg
-
SEX/DURATION :
-
female 20-40 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
MUTATION DATA
-
TYPE OF TEST :
-
DNA adduct
-
TEST SYSTEM :
-
Rodent - rat Liver
-
DOSE/DURATION :
-
50 umol/L
-
REFERENCE :
-
MUREAV Mutation Research. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1964- Volume(issue)/page/year: 345,181,1995
|