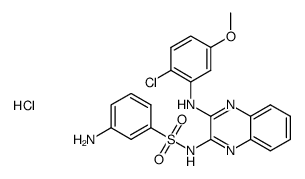
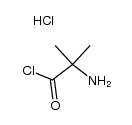
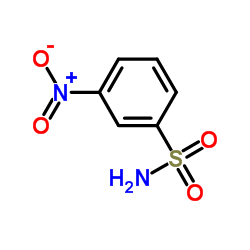
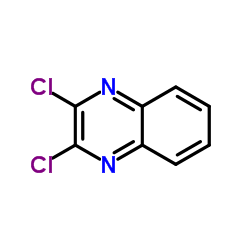
934526-89-3
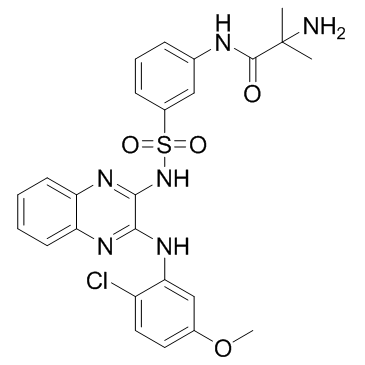
| Name | 2-amino-N-[3-[[3-(2-chloro-5-methoxyanilino)quinoxalin-2-yl]sulfamoyl]phenyl]-2-methylpropanamide |
|---|---|
| Synonyms |
unii-60es45ktmk
pilaralisib XL 147 XL-147 |
| Description | Pilaralisib (XL147; SAR245408) is a potent and highly selective class I PI3Ks inhibitor with IC50s of 39 nM, 383 nM, 23 nM and 36 nM for PI3Kα, PI3Kβ, PI3Kγ, and PI3Kδ. |
|---|---|
| Related Catalog | |
| Target |
PI3Kα:39 nM (IC50) PI3Kβ:383 nM (IC50) PI3Kδ:36 nM (IC50) PI3Kγ:23 nM (IC50) Vps34:6974 nM (IC50) DNA-PK:4750 nM (IC50) |
| In Vitro | Pilaralisib (XL147) displays potent inhibitory activity against Class I PI3K isoforms p110α, p110δ, and p110γ, with IC50s of 39, 36, and 23 nM, respectively. Pilaralisib (XL147) is less potent against the remaining Class I isoform, p110β, with an IC50 value of 383 nM. The IC50 value for inhibition of PI3Kα by Pilaralisib (XL147) is determined at various concentrations of ATP, revealing XL147 to be an ATP-competitive inhibitor with an equilibrium inhibition constant (Ki) value of 42 nM. Pilaralisib (XL147) has relatively weak inhibitory activity toward the class III PI3K vacuolar sorting protein 34 (VPS34; IC50 value of ~7.0 M) and the PI3K-related DNA-dependent protein kinase (DNA-PK; IC50 value of 4.75 μM). In an mTOR kinase immunoprecipitation assay using cell lysates, Pilaralisib (XL147) does not inhibit mTOR activity toward the physiologic substrate protein eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1; IC50>15 μM). Consistent with its inhibitory activity against purified PI3K proteins, Pilaralisib (XL147) inhibits EGF-induced PIP3 production in PC-3 and MCF7 cells in serum-free medium with IC50s of 220 and 347 nM, respectively. The ability of Pilaralisib (XL147) to inhibit phosphorylation of key signaling proteins downstream of PI3K is examined by assessing its effects on EGF-stimulated phosphorylation of AKT and on nonstimulated phosphorylation of S6 in PC-3 cells in serum-free media by cell-based ELISA. Pilaralisib (XL147) inhibits these activities with IC50s of 477 and 776 nM, respectively[1]. |
| In Vivo | The ability of Pilaralisib (XL147) to inhibit this endogenous phosphorylation of AKT, p70S6K, and S6 is examined following a single oral dose of 10, 30, 100, or 300 mg/kg. The tumors are harvested 4, 24, or 48 hours postdose and homogenized in lysis buffer. Tumor lysates from each animal (n=4) are then pooled for each group and analyzed for levels of total and phosphorylated AKT, p70S6K, and S6 by Western immunoblotting. Administration of Pilaralisib (XL147) causes a dose-dependent decrease in phosphorylation of AKT, p70S6K, and S6 in the tumors, reaching a maximum of 81% inhibition of AKT phosphorylation at 300 mg/kg at 4 hours. The dose-response relationships derived from the 4-hour time point predict 50% inhibition of AKT, p70S6K, and S6 phosphorylation at doses of approximately 100 mg/kg (pAKTT308), 54 mg/kg (pAKTS473), 71 mg/kg (p-p70S6K), and 103 mg/kg (pS6). The inhibition of AKT, p70S6K, and S6 phosphorylation in MCF7 tumors following a 100 mg/kg dose of Pilaralisib (XL147) is maximal at 4 hours, reaching 55% to 75%; however, the level of inhibition decreased to 8% to 45% by 24 hours, and only minimal or no inhibition was evident by 48 hours. Following a 300 mg/kg dose of Pilaralisib (XL147), inhibition is also maximal at 4 hours (65%-81%). However, in contrast with the 100 mg/kg dose, inhibition at 24 hours (51%-78%) is almost comparable with that seen at 4 hours, and partial inhibition (25%-51%) persisted through 48 hours[1]. |
| Cell Assay | MCF7 human mammary carcinoma cells and PC-3 human prostate adenocarcinoma cells are maintained in culture conditions at 37°C under 5% CO2. For PI3K pathway status assessment following EGF treatment, the culture medium is replaced with test compounds dissolved in serum-free DMEM containing 0.3% DMSO. After incubation for 3 hours, cells are stimulated with 100 ng/mL of EGF for 10 minutes and Western immunoblot analysis of cell lysates is performed. Assessment of mTOR pathway status in Ramos cells is performed. Cellular proliferation is assessed using the Cell Proliferation ELISA, bromodeoxyuridine (BrdUrd) chemiluminescence kit. Cytotoxicity, apoptosis (caspases-3/7), anchorage-independent growth, and PC-3 cell migration assays are performed. Hepatocyte growth factor (HGF)-induced chemotaxis is assessed. Theendothelial cell tube formation assay is performed, with minor modifications. Total tube length is quantified with Image Pro Plus software[1]. |
| Animal Admin | Mice[1] Female athymic nude mice and male nude mice are used. Tumor cells are cultured and established as xenografts in mice, and body and tumor weights assessed. Statistical significance is determined using the two-tailed Student ttest (significance defined as P<0.05). Pilaralisib (XL147) is formulated in sterile water/10 mmol/L HCl or water and administered at the indicated doses and regimens by oral gavage at a dose volume of 10 mL/kg. |
| References |
| Molecular Formula | C25H25ClN6O4S |
|---|---|
| Molecular Weight | 541.02200 |
| Exact Mass | 540.13500 |
| PSA | 166.66000 |
| LogP | 5.78640 |
| Storage condition | -20°C |
|
~% 
934526-89-3 |
| Literature: SANOFI; BAILLON, Bruno; BAULIER, Virginie; COMTE, Myriam; FUGIER, Matthieu; KOZLOVIC, Stéphane; PERRIN, Marc-Antoine Patent: WO2014/41144 A1, 2014 ; Location in patent: Paragraph 00172; 00173; 00174 ; |
|
~% 
934526-89-3 |
| Literature: SANOFI; BAILLON, Bruno; BAULIER, Virginie; COMTE, Myriam; FUGIER, Matthieu; KOZLOVIC, Stéphane; PERRIN, Marc-Antoine Patent: WO2014/41144 A1, 2014 ; |
|
~% 
934526-89-3 |
| Literature: SANOFI; BAILLON, Bruno; BAULIER, Virginie; COMTE, Myriam; FUGIER, Matthieu; KOZLOVIC, Stéphane; PERRIN, Marc-Antoine Patent: WO2014/41144 A1, 2014 ; |
|
~% 
934526-89-3 |
| Literature: SANOFI; BAILLON, Bruno; BAULIER, Virginie; COMTE, Myriam; FUGIER, Matthieu; KOZLOVIC, Stéphane; PERRIN, Marc-Antoine Patent: WO2014/41144 A1, 2014 ; |