6631-94-3
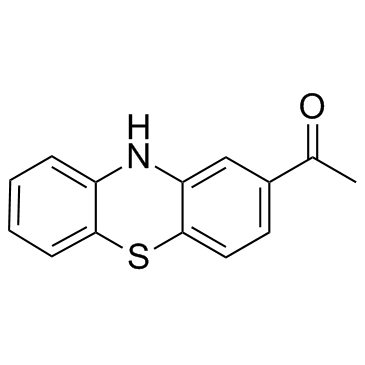
| Name | 2-Acetylphenothiazine |
|---|---|
| Synonyms |
2-acetyl-1-phenothiazine
EINECS 229-626-4 2-Acetyl-10H-phenothiazine 1-phenothiazin-2-yl-ethanone 2-acetyl-phenothiazine MFCD00005017 1-(10H-phenothiazin-2-yl)ethanone methyl phenothiazin-2-yl ketone ML171 |
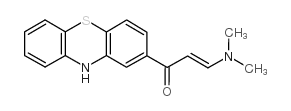
| Description | ML171 (2-Acetylphenothiazine;2-APT) is a potent and selective Nox1 inhibitor that blocks Nox1-dependent ROS generation, with an IC50 of 0.25 μM in HEK293-Nox1 confirmatory assay. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.25 μM (HEK293-Nox1), 0.129 μM (HT29)[1] |
| In Vitro | Nox1-dependent ROS generation has been shown to play a pivotal role in cell signaling, cell growth, angiogenesis, motility and blood pressure regulation. ML171 strongly blocks ROS generation in HT29 cells (IC50=0.129 μM) and only increasing over-expression of Nox1 can overcome the blockage of ROS generation caused by ML171 treatment in HEK293 cell system reconstituted with all the components required Nox1-dependent ROS generation. ML171 efficiently blocks ROS production measured by carboxy-H2-DCFDA staining as well as DPI used as a positive control. When ML171 is tested in HEK293-Nox1 reconstituted cell system, higher potency in blocking Nox1-dependent ROS generation is observed compared with the parental compound[1]. |
| Cell Assay | HT29 cells are cultured in 150 mm diameter plate and when 70-80% confluence is reached, cells are trypsinized, harvested in HBSS and counted. 4×104 cells are dispensed into individual wells in 30 μL final volume (384 well plates) by using a robotic liquid handler. Cells are treated for 60 min at 37°C with 50 nL of DPI, DMSO and library compounds (including ML171) which are automatically dispensed into individual wells from their respective assay plates. This will correspond to a final concentration of 10 μM DPI or library compounds (ML171), and 0.1% DMSO. 20 μL of a mixture containing 200 μM luminol plus 0.32 units of HRP (final concentration) is added. Luminescence is quantified using a 384-well plate luminometer. The data output consisting of the emission intensities for each well is imported into a spread-sheet program (such as Excel) for further processing. As designed, compounds that inhibit Nox1 activity will reduce cellular ROS production, leading to reduced probe-ROS interactions and reduced well luminescence. Compounds are considered ‘hits’ and further processed when light emission is blocked >75% 7 than DMSO wells (DMSO and DPI wells are set to 0% and 100% respectively). Compounds are tested in singlicate at a concentration of 10 μM[1]. |
| References |
| Density | 1.249 g/cm3 |
|---|---|
| Boiling Point | 455.7ºC at 760 mmHg |
| Melting Point | 180-185 °C(lit.) |
| Molecular Formula | C14H11NOS |
| Molecular Weight | 241.30800 |
| Flash Point | 229.4ºC |
| Exact Mass | 241.05600 |
| PSA | 54.40000 |
| LogP | 4.23540 |
| Index of Refraction | 1.653 |
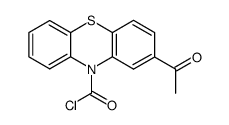
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2934300000 |
|---|---|
| Summary | 2934300000. other compounds containing in the structure a phenothiazine ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |


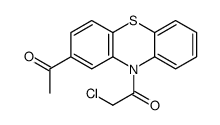


![2-Acetyl-10-[3-(4-methylpiperazino)propyl]-10H-phenothiazine structure](https://image.chemsrc.com/caspic/095/1053-74-3.png)
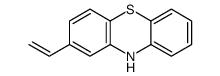
![1-[10-(3-chloropropyl)-10H-phenothiazin-2-yl]ethan-1-one structure](https://image.chemsrc.com/caspic/486/39481-55-5.png)