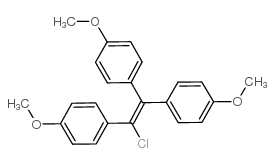
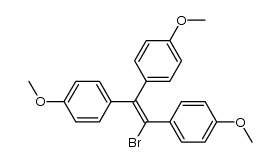
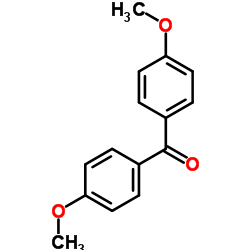
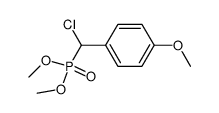
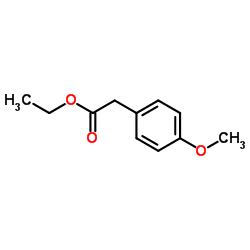
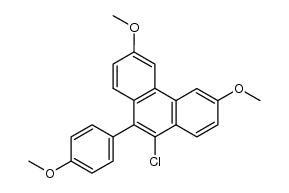
569-57-3
| Name | chlorotrianisene |
|---|---|
| Synonyms |
1-chloro-1,2,2-tris(p-methoxyphenyl)ethene
TACE 1,1',1''-(1-chloro-1-ethenyl-2-ylidene)tris[4-methoxy-benzene] Chlortrianisestrol Merbentul Chlorestrolo Chlorotrianisene Tris-(4-methoxy-phenyl)-vinylchlorid chloro-tris-(4-methoxy-phenyl)-ethene Chlorotrianizen Chlor-tris-(4-methoxy-phenyl)-aethen Chlortrianisen Chloortrianisestrol 1-[1-chloro-2,2-bis(4-methoxyphenyl)ethenyl]-4-methoxybenzene Chlorotrianisine Chlortrianizen 2-Chlor-1,1,2-tris-(4-methoxy-phenyl)-aethylen |
| Description | Chlorotrianisene is a long-acting non-steroidal estrogen and an orally active estrogen receptor modulator. Chlorotrianisene exhibits antiestrogenic activity. Chlorotrianisene potently inhibits the enzyme COX-1 and inhibits platelet aggregation in whole blood[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Estrogen receptor[1] COX-1[2] |
| In Vitro | Comparison of intracellular estrogen receptor (ER) affinities of Chlorotrianisene with respective rat uterine cytosolic ER affinities has initially suggested the potential for activation of ER as a mechanism of growth stimulation. Chlorotrianisene exhibits concentration dependent cell growth stimulation with an EC50 of 28 nM and a Ki of 500 nM in MCF-7 cells[1]. |
| In Vivo | The incubation of Chlorotrianisene with rat liver microsomes and NADPH generates a reactive intermediate which binds covalently to proteins. Intermediate may inactivate the uterine estrogen receptors (ER). The incubation of Chlorotrianisene with rat liver microsomes and NADPH in the presence of rat uteri, under conditions which generate intermediate, markedly decreased the binding capacity of the ER for [3H]estradiol (E2)[3]. |
| References |
| Density | 1.168g/cm3 |
|---|---|
| Boiling Point | 514.2ºC at 760mmHg |
| Melting Point | 114-116ºC |
| Molecular Formula | C23H21ClO3 |
| Molecular Weight | 380.86400 |
| Flash Point | 164.1ºC |
| Exact Mass | 380.11800 |
| PSA | 27.69000 |
| LogP | 5.86780 |
| Index of Refraction | 1.591 |
| Storage condition | -70°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| WGK Germany | 3 |
|---|---|
| RTECS | KV0600000 |
| Precursor 10 | |
|---|---|
| DownStream 3 | |