10361-93-0
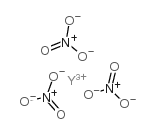
| Name | yttrium(3+),trinitrate |
|---|---|
| Synonyms |
Yttrium nitrate
Yttrium(3+) nitrate Nitric acid,yttrium(3+) salt Yttrium trinitrate yttrium(3+) trinitrate Yttrium(III) nitrate EINECS 233-802-6 |
| Molecular Formula | N3O9Y |
|---|---|
| Molecular Weight | 274.92100 |
| Exact Mass | 274.86900 |
| PSA | 206.64000 |
| LogP | 0.85230 |
|
Section 1. Chemical Product and Company Identification Yttrium Nitrate, hydrate Common Name/ Trade Name Manufacturer Commercial Name(s) Synonym
Chemical Name Chemical Family Yttrium Nitrate, hydrate Section 4. First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Cold water may be used. Get medical attention. Skin ContactIn case of contact, immediately flush skin with plenty of water. Cover the irritated skin with an emollient. Remove contaminated clothing and shoes. Cold water may be used.Wash clothing before reuse. Thoroughly clean shoes before reuse. Get medical attention. Serious Skin ContactWash with a disinfectant soap and cover the contaminated skin with an anti-bacterial cream. Seek medical attention. InhalationIf inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention. Serious InhalationEvacuate the victim to a safe area as soon as possible. Loosen tight clothing such as a collar, tie, belt or waistband. If breathing is difficult, administer oxygen. If the victim is not breathing, perform mouth-to-mouth resuscitation. Seek medical attention. IngestionDo NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. If large quantities of this material are swallowed, call a physician immediately. Loosen tight clothing such as a collar, tie, belt or waistband. Serious IngestionNot available. Section 5. Fire and Explosion Data Flammability of the Product Non-flammable. Auto-Ignition Temperature Not applicable. Flash PointsNot applicable. Flammable LimitsNot applicable. Products of CombustionNot available. Fire Hazards in Presence of of combustible materials of organic materials Various Substances Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available. Risks of explosion of the product in presence of static discharge: Not available. of Various Substances Not applicable. Fire Fighting Media and Instructions Special Remarks onDanger! Oxidizer! Contact with other material may cause fire. Contact with combustible or organic materials may cause fire. Fire Hazards Special Remarks on Explosion Not available. Hazards Section 6. Accidental Release Measures Small SpillUse appropriate tools to put the spilled solid in a convenient waste disposal container. Large Spill Oxidizing material. Stop leak if without risk. Avoid contact with a combustible material (wood, paper, oil, clothing...). Keep substance damp using water spray. Do not touch spilled material. Prevent entry into sewers, basements or confined areas; Yttrium Nitrate, hydrate Section 7. Handling and Storage PrecautionsKeep away from heat. Keep away from sources of ignition. Keep away from combustible material.. Do not breathe dust. Wear suitable protective clothing. If you feel unwell, seek medical attention and show the label when possible. Avoid contact with skin and eyes. Keep away from incompatibles such as combustible materials, organic materials, alkalis. StorageKeep container tightly closed. Keep container in a cool, well-ventilated area. Separate from acids, alkalies, reducing agents and combustibles. See NFPA 43A, Code for the Storage of Liquid and Solid Oxidizers. Section 8. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSplash goggles. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used a Large Spillto avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Exposure LimitsNot available. Section 9. Physical and Chemical Properties Physical state and appearance Solid. (Crystalline solid.)OdorOdorless. Not available. Taste Molecular Weight274.94 g/mole White. Color Not available. pH (1% soln/water) Boiling PointNot available. Melting PointNot available. Critical TemperatureNot available. Specific GravityNot available. Not applicable. Vapor Pressure Vapor DensityNot available. Not available. Volatility Odor ThresholdNot available. Water/Oil Dist. Coeff.Not available. Ionicity (in Water)Not available. Dispersion PropertiesSee solubility in water. SolubilitySoluble in cold water, hot water. Section 10. Stability and Reactivity Data StabilityThe product is stable. Instability TemperatureNot available. Conditions of InstabilityDust generation, incompatibles Incompatibility with variousHighly reactive with combustible materials, organic materials. substancesReactive with alkalis. CorrosivityNot available. Yttrium Nitrate, hydrate Special Remarks onOxidizer ReactivityIncompatible with strong bases, easily oxidized materials, organic materials, flammable substances. Special Remarks onNot available. Corrosivity PolymerizationWill not occur. Section 11. Toxicological Information Routes of EntryInhalation. Ingestion. Toxicity to AnimalsLD50: Not available. LC50: Not available. Chronic Effects on Humans May cause damage to the following organs: blood. Other Toxic Effects onHazardous in case of skin contact (irritant), of ingestion. HumansSlightly hazardous in case of inhalation. Special Remarks onNot available. Toxicity to Animals Special Remarks onMay cause adverse reproductive effects based on animal data. Chronic Effects on HumansMay affect genetic material based on animal data. May cause cancer (tumorigenic) based on animal data. Special Remarks on otherAcute Potential Health Effects: Toxic Effects on HumansSkin: Causes moderate skin irritation. Eyes: Causes moderate to severe eye irritation. Inhalation: Dust may cause respiratory tract irritation, chemical pneumonia, shortness of breath and wheezing. Ingestion: May be harmful if ingested. May cause gastrointestinal tract irritation with nausea, vomiting and possible holes in the esophagus. The first clinical signs associated with nitrate poisoning include: Gastroenteritis, abdominal pain, nausea, vomiting(spontaneous vomiting), diarrhea, metabolic acidosis. Purging and diuresis can be expected. The toxicity of nitrates is due to the in vivo conversion to nitrites. The primary toxic effects of nitrites include orthostatic hypotension (due to perpheral vasodilation) and methemoglobinemia (the formation of methemoglobin in the blood which causes deficient oxygenation of the blood due to decreased available hemoglobin). Other symptoms may include muscular weakness, dizziness, lightheadness, fatigue, throbbing headache, general depression, mental impairment, incoordination, seizures convulsions, bradycardia or tachydardia (slow or fast heart beat), dysrhythmias, dyspnea. Furthermore, methemoglobinemia due to inadequate oxygenation of the blood can lead to progressive cyanosis, and coma. Cyanosis is first visible as a bluish discoloration of the mucous membranes and unpigmented areas of the body. Chronic Potential Health Effects: Ingestion: Under some circumstances methemoglobinemia occurs individuals when the nitrate is converted by bacteria in the stomach to the nitrite. Nausea, vomiting, dizziness, rapid or slow heart beat, irregular breathing, convulsions, symptoms similar to acute ingestion, coma and death can occur should this conversion take place. Repeated or prolonged ingestion may also affect the liver and cause anorexia (weight loss). Section 12. Ecological Information EcotoxicityNot available. BOD5 and CODNot available. Products of BiodegradationPossibly hazardous short term degradation products are not likely. However, long term degradation products may arise. Toxicity of the ProductsThe product itself and its products of degradation are not toxic. of Biodegradation Special Remarks on theNot available. Products of Biodegradation Yttrium Nitrate, hydrate Section 13. Disposal Considerations Waste DisposalWaste must be disposed of in accordance with federal, state and local environmental control regulations. Section 14. Transport Information DOT ClassificationCLASS 5.1: Oxidizing material. : Nitrate, Inorganic, n.o.s (Yttrium Nitrate) UNNA: 1477 PG: III Identification Not available. Special Provisions for Transport DOT (Pictograms) OXIDIZER 5.1 Section 15. Other Regulatory Information and Pictograms TSCA 8(b) inventory: Yttrium Nitrate Federal and State Regulations CaliforniaCalifornia prop. 65: This product contains the following ingredients for which the State of California has found to cause cancer which would require a warning under the statute: No products were found. Proposition 65 Warnings California prop. 65: This product contains the following ingredients for which the State of California has found to cause birth defects which would require a warning under the statute: No products were found. Other RegulationsOSHA: Hazardous by definition of Hazard Communication Standard (29 CFR 1910.1200). EINECS: This product is on the European Inventory of Existing Commercial Chemical Substances. CLASS C: Oxidizing material. Other ClassificationsWHMIS (Canada) CLASS D-2B: Material causing other toxic effects (TOXIC). DSCL (EEC) R8- Contact with combustible materialS26- In case of contact with eyes, rinse may cause fire.immediately with plenty of water and seek R36/38- Irritating to eyes and skin.medical advice. S36- Wear suitable protective clothing. S46- If swallowed, seek medical advice immediately and show this container or label. Health Hazard HMIS (U.S.A.)2 National Fire Protection 0 Flammability 0 Association (U.S.A.) Fire Hazard 2 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E WHMIS (Canada) (Pictograms) DSCL (Europe) (Pictograms) Yttrium Nitrate, hydrate TDG (Canada) (Pictograms) 5.1 ADR (Europe) (Pictograms) Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Wear appropriate respirator when ventilation is inadequate. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| RIDADR | UN 1477 |
|---|