475108-18-0
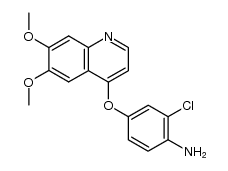
| Name | 1-[2-chloro-4-(6,7-dimethoxyquinolin-4-yl)oxyphenyl]-3-(5-methyl-1,2-oxazol-3-yl)urea |
|---|---|
| Synonyms |
AV951
AV-951 Urea, N-[2-chloro-4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-N'-(5-methyl-3-isoxazolyl)- N-(2-chloro-4-((6,7-dimethoxy-4-quinolyl)oxy)phenyl)-N'-(5-methyl-3-isoxazolyl)urea Tivozanib KRN-951 1-{2-Chloro-4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl}-3-(5-methyl-1,2-oxazol-3-yl)urea |
| Description | Tivozanib (AV-951; KRN951) is a highly potent and selective VEGFR 1/2/3 inhibitor with IC50s of 0.21, 0.16, and 0.24 nM in cell assay, respectively. |
|---|---|
| Related Catalog | |
| Target |
VEGFR1:30 nM (IC50) VEGFR2:6.5 nM (IC50) VEGFR3:15 nM (IC50) |
| In Vitro | Tivozanib potently inhibits VEGF-induced VEGFR2 phosphorylation in endothelial cells (IC50=0.16 nM). It also inhibits ligand-induced phosphorylation of PDGFRβ and c-Kit (IC50=1.72 and 1.63 nM, respectively). Tivozanib blocks VEGF-dependent, but not VEGF-independent, activation of mitogenactivated protein kinases and proliferation of endothelial cells. It inhibits VEGF-mediated migration of human umbilical vein endothelial cells[1]. |
| In Vivo | Following p.o. administration to athymic rats, Tivozanib decreases the microvessel density within tumor xenografts and attenuates VEGFR-2 phosphorylation levels in tumor endothelium. It also displays antitumor activity against a wide variety of human tumor xenografts, including lung, breast, colon, ovarian, pancreas, and prostate cancer[1]. |
| Kinase Assay | Cell-free kinase assays are done in quadruplicate with 1 μM ATP to determine the IC50 values of KRN951 against a variety of recombinant receptor and nonreceptor tyrosine kinases[1]. |
| Cell Assay | Cell-based assays are done to determine the ability of KRN951 to inhibit ligand-dependent phosphorylation of receptor tyrosine kinases. Briefly, the cells are starved overnight in appropriate basic medium containing 0.5% fetal bovine serum (FBS). Following the addition of KRN951 or 0.1% DMSO, the cells are incubated for 1 hour and then stimulated with the cognate ligand at 37°C. Receptor phosphorylation is induced for 5 minutes except for VEGFR3 (10 minutes), c-Met (10 minutes), and c-Kit (15 minutes). All the ligands used in the assays are human recombinant proteins, except for VEGF-C, a rat recombinant protein. Following cell lysis, receptors are immunoprecipitated with appropriate antibodies and subjected to immunoblotting with phosphotyrosine. Quantification of the blots and calculation of IC50 values are carried out[1]. |
| Animal Admin | Mice: Cancer cells are s.c. inoculated into the right flank of the athymic rats. Once established, tumors of 1,500 mm3 are surgically excised and smaller tumor fragments (20-30 mg) are s.c. implanted in the right flank of irradiated rats. Oral administration of KRN951 (0.2 or 1 mg/kg) or vehicle is initiated at the day of randomization (day 0). Tumor volume is measured twice weekly with Vernier calipers, and calculated[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 550.4±50.0 °C at 760 mmHg |
| Molecular Formula | C22H19ClN4O5 |
| Molecular Weight | 454.863 |
| Flash Point | 286.7±30.1 °C |
| Exact Mass | 454.104401 |
| PSA | 107.74000 |
| LogP | 4.31 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.680 |
| Storage condition | -20°C |
|
~85% 
475108-18-0 |
| Literature: EP1559715 A1, ; Page/Page column 20 ; |
| Precursor 3 | |
|---|---|
| DownStream 1 | |

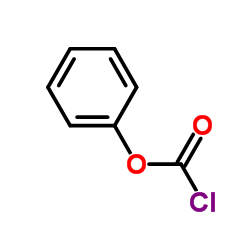
![1-[2-chloro-4-(6,7-dimethoxyquinolin-4-yl)oxyphenyl]-3-(5-methyl-1,2-oxazol-3-yl)urea,hydrochloride structure](https://image.chemsrc.com/caspic/406/682745-43-3.png)