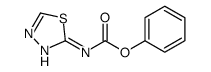
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
XI3000000
-
CHEMICAL NAME :
-
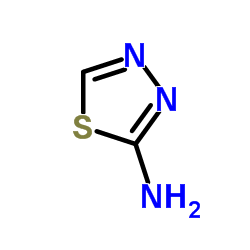
1,3,4-Thiadiazole, 2-amino-
-
CAS REGISTRY NUMBER :
-
4005-51-0
-
BEILSTEIN REFERENCE NO. :
-
0107135
-
LAST UPDATED :
-
199712
-
DATA ITEMS CITED :
-
15
-
MOLECULAR FORMULA :
-
C2-H3-N3-S
-
MOLECULAR WEIGHT :
-
101.14
-
WISWESSER LINE NOTATION :
-
T5NN DSJ CZ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
200 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - muscle weakness Gastrointestinal - hypermotility, diarrhea Skin and Appendages - hair
-
REFERENCE :
-
TAKHAA Takeda Kenkyusho Ho. Journal of the Takeda Research Laboratories. (Takeda Yakuhin Kogyo K.K., 2-17-85 Jusohon-machi, Yodogawa-ku, Osaka 532, Japan) V.29- 1970- Volume(issue)/page/year: 35,68,1976
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
200 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JHMJAX Johns Hopkins Medical Journal. (Baltimore, MD) V.120-151, 1967-82. Discontinued. Volume(issue)/page/year: 130,95,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
6500 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - muscle weakness Gastrointestinal - hypermotility, diarrhea Skin and Appendages - hair
-
REFERENCE :
-
TAKHAA Takeda Kenkyusho Ho. Journal of the Takeda Research Laboratories. (Takeda Yakuhin Kogyo K.K., 2-17-85 Jusohon-machi, Yodogawa-ku, Osaka 532, Japan) V.29- 1970- Volume(issue)/page/year: 35,68,1976 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
20 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
REFERENCE :
-
YHHPAL Yaoxue Xuebao. Acta Pharmaceutica Sinica. Pharmaceutical Journal. (China International Book Trading Corp., POB 2820, Beijing, Peop. Rep. China) V.1- 1953- Suspended 1966-78. Volume(issue)/page/year: 16,654,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
REFERENCE :
-
CPHPA5 Jiepou Xuebao. Journal of Anatomy. (China International Book Trading Corp., POB 2820, Beijing, Peop. Rep. China) V.1-9, 1953-66; V.10- 1979- Volume(issue)/page/year: 12,212,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
20 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
CPHPA5 Jiepou Xuebao. Journal of Anatomy. (China International Book Trading Corp., POB 2820, Beijing, Peop. Rep. China) V.1-9, 1953-66; V.10- 1979- Volume(issue)/page/year: 12,212,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
100 mg/kg
-
SEX/DURATION :
-
female 16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - hepatobiliary system Reproductive - Effects on Newborn - biochemical and metabolic
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 27,29A,1983
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
1600 mg/kg
-
SEX/DURATION :
-
female 1-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 5,250,1972
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
1600 mg/kg
-
SEX/DURATION :
-
female 1-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 5,250,1972
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
100 mg/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - body wall Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
JEEMAF Journal of Embryology and Experimental Morphology. (Essex, UK) V.1-98, 1953-86. For Publisher information, see DEVPED Volume(issue)/page/year: 30,257,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
100 mg/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Specific Developmental Abnormalities - gastrointestinal system
-
REFERENCE :
-
JEEMAF Journal of Embryology and Experimental Morphology. (Essex, UK) V.1-98, 1953-86. For Publisher information, see DEVPED Volume(issue)/page/year: 30,257,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
175 mg/kg
-
SEX/DURATION :
-
female 10-11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
JHMJAX Johns Hopkins Medical Journal. (Baltimore, MD) V.120-151, 1967-82. Discontinued. Volume(issue)/page/year: 130,95,1972
|