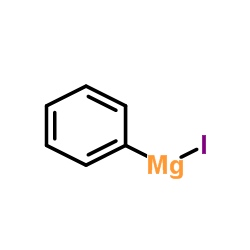
16002-63-4
| Name | Phenylmagnesium Iodide |
|---|---|
| Synonyms |
Phenyl lithium
Magnesium, iodophenyl- Lithiobenzene LITHIUMPHENYL phenyllithium solution phenylllithium phenyllithium,1.5-1.7m in cyclohexane/ether PHENYLLITHIUM DIBUTYL PHLI phenyllitihium Iodo(phenyl)magnesium phenyl-lithiu PHENYLLITHIUM SOLUTION IN DIBUTYLETHER phenylmagnesium iodide solutioninDibutylether |
| Molecular Formula | C6H5IMg |
|---|---|
| Molecular Weight | 228.313 |
| Exact Mass | 227.928619 |
| LogP | 2.37250 |
|
Section I.Chemical Product and Company Identification Chemical NamePhenylmagnesium Iodide (42% in Ethyl Ether, ca. 2mol/L) Portland OR SynonymMagnesium, Iodophenyl- (9 CI) Chemical FormulaC6H5MgI CAS Number16002-63-4
60-29-7 (Ethyl Ether) Section II.Composition and Information on Ingredients CAS Number Percent (%)TLV/PELToxicology Data Chemical Name Phenylmagnesium Iodide42.0 Not available.Ethyl Ether: 16002-63-4 (42% in Ethyl Ether, ca. 2mol/L) 60-29-7 (Ethyl58.0Rat LD50 (oral) 1215mg/kg Rabbit LD50 (dermal) >20ml/kg Ether) Mouse LC50 (inhalation) 31000ppm/30M Section III. Hazards Identification Acute Health EffectsCorrosive to skin, eyes, and respiratory system. Liquid or spray mist may produce tissue damage, particularly in mucous membranes of the eyes, mouth and respiratory tract. Skin contact may produce burns. Eye contact can result in corneal damage or blindness. Inhalation of the spray mist may produce severe irritation of respiratory tract, characterized by coughing, choking, or shortness of breath. Corrosive materials may cause serious injury if ingested. Harmful if ingested or inhaled. Minimize exposure to this material. Severe overexposure can result in injury or death. Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound. Chronic Health EffectsCARCINOGENIC EFFECTS : Not available. MUTAGENIC EFFECTS : Not available. TERATOGENIC EFFECTS : Not available. DEVELOPMENTAL TOXICITY: Not available. Repeated or prolonged contact with spray mist may produce chronic eye irritation and severe skin irritation. Repeated or prolonged exposure to spray mist may produce respiratory tract irritation leading to frequent attacks of bronchial infection. Section IV.First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical attention. Skin ContactIn case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing before reuse. Thoroughly clean shoes before reuse. Get medical attention. InhalationIf the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not improve. IngestionDO NOT INDUCE VOMITING. Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic material was ingested; the absence of such signs, however, is not conclusive. Continued on Next Page Phenylmagnesium Iodide (42% in Ethyl Ether, ca. 2mol/L) Section V.Fire and Explosion Data Auto-Ignition160°C (320°F) (Ethyl Ether) FlammabilityFlammable. Flash PointsFlammable LimitsNot available. -40°C (-40°F) (Ethyl Ether). Combustion ProductsThese products are toxic carbon oxides (CO, CO 2). Some metallic oxides. Fire Hazards Not available. Risks of explosion of the product in presence of mechanical impact: Not available. Explosion Hazards Risks of explosion of the product in presence of static discharge: Not available. Fire Fighting Media Flammable liquid. SMALL FIRE: Use DRY chemical powder. and Instructions LARGE FIRE: Use alcohol foam, water spray or fog. Consult with local fire authorities before attempting large scale fire-fighting operations. Section VI.Accidental Release Measures Spill CleanupWater-reactive material. Flammable material. Corrosive material. Harmful material. May form explosive peroxides. Air, heat, and light sensitive material. Do not distill to dryness. Refrigerate material. Instructions Keep away from heat. Mechanical exhaust required. Stop leak if without risk. Absorb with DRY earth, sand or other non-combustible material. DO NOT get water inside container. DO NOT touch spilled material. Use water spray curtain to divert vapor drift. Prevent entry into sewers, basements or confined areas; dike if needed. Consult federal, state, and/or local authorities for assistance on disposal. Section VII. Handling and Storage Handling and StorageWATER-REACTIVE. FLAMMABLE. CORROSIVE. HARMFUL. MAY FORM EXPLOSIVE PEROXIDES. AIR, HEAT, AND LIGHT SENSITIVE. DO NOT DISTILL TO DRYNESS. REFRIGERATE. Keep under inert atmosphere. Keep Information container dry. Do not breathe gas/fumes/ vapor/spray. Never add water to this product. Wear suitable protective clothing. If you feel unwell, seek medical attention and show the label when possible. Treat symptomatically and supportively. Always store away from incompatible compounds such as oxidizing agents, acids. Section VIII. Exposure Controls/Personal Protection Engineering ControlsProvide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapors below their respective threshold limit value. Ensure that eyewash station and safety shower is proximal to the work-station location. Personal ProtectionFace shield. Lab coat. Vapor respirator. Boots. Gloves. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Be sure to use a MSHA/NIOSH approved respirator or equivalent. Exposure LimitsNot available. Section IX. Physical and Chemical Properties Physical state @ 20°CLiquid.Solubility Ethyl Ether: Miscible with lower aliphatic alcohols, Specific GravityNot available.benzene, chloroform, petroleum ether, fat solvents, many oils, most organic solvents. Soluble in concentrated hydrochloric acid, acetone, solvent naphtha. Very soluble in ethanol, ethyl ether. In water, 6.04X10+4mg/L @ 25°C. Molecular Weight228.31Partition CoefficientNot available. Boiling Point34.6°C (94.3°F) (Ethyl Ether)Vapor Pressure59 kPa (@ 20°C) (Ethyl Ether) Melting Point-116°C (-176.8°F) (Ethyl Ether)Vapor Density2.6 (Air = 1) (Ethyl Ether) 1.3526 @ 20°C (Ethyl Ether)VolatilityNot available. Refractive Index Critical TemperatureOdorEther-like. Not available. Dynamic: 0.2448 cP (Ethyl Ether)Burning and sweet. ViscosityTaste Continued on Next Page Phenylmagnesium Iodide (42% in Ethyl Ether, ca. 2mol/L) Section X.Stability and Reactivity Data Stability This material is stable if stored under proper conditions. (See Section VII for instructions) Conditions of InstabilityHeat and light accelerate peroxide formation. Avoid excessive heat and light. Incompatibilities Reactive with oxidizing agents, strong acids. The product REACTS violently with water to emit FLAMMABLE BUT NON TOXIC GASES. Section XI. Toxicological Information RTECS NumberKI5775000 (Ethyl Ether) Eye Contact. Ingestion. Inhalation. Skin contact. Routes of Exposure Toxicity DataEthyl Ether: Rat LD50 (oral) 1215mg/kg Rabbit LD50 (dermal) >20ml/kg Mouse LC50 (inhalation) 31000ppm/30M CARCINOGENIC EFFECTS : Not available. Chronic Toxic Effects MUTAGENIC EFFECTS : Not available. TERATOGENIC EFFECTS : Not available. DEVELOPMENTAL TOXICITY: Not available. Repeated or prolonged contact with spray mist may produce chronic eye irritation and severe skin irritation. Repeated or prolonged exposure to spray mist may produce respiratory tract irritation leading to frequent attacks of bronchial infection. Acute Toxic EffectsCorrosive to skin, eyes, and respiratory system. Liquid or spray mist may produce tissue damage, particularly in mucous membranes of the eyes, mouth and respiratory tract. Skin contact may produce burns. Eye contact can result in corneal damage or blindness. Inhalation of the spray mist may produce severe irritation of respiratory tract, characterized by coughing, choking, or shortness of breath. Corrosive materials may cause serious injury if ingested. Harmful if ingested or inhaled. Minimize exposure to this material. Severe overexposure can result in injury or death. Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound. Section XII.Ecological Information EcotoxicityNot available. Environmental FateEthyl Ether: Diethyl ether's production and use as a solvent, in the manufacture of gun powder, and as a primer for gasoline engines may result in its release to the environment through various waste streams. If released to air, a vapor pressure of 538 mm Hg at 25 deg C indicates diethyl ether will exist solely as a vapor in the ambient atmosphere. Vapor-phase diethyl ether will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals and nitrate radicals; half-lives for these reactions in air are estimated to be 1.2 and 5.8 days, respectively. Direct photolysis is not expected to be an important removal process since aliphatic ethers do not absorb light in the environmental spectrum. If released to soil, diethyl ether is expected to have high mobility based upon an estimated Koc of 73. Volatilization from moist soil surfaces is expected to be an important fate process based upon a Henry's Law constant of 1.23X10-3 atm-cu m/mole. Diethyl ether is expected to volatilize from dry soil surfaces based upon its vapor pressure. Aqueous screening studies indicate biodegradation is expected to be a slow fate process in both soil and water; 0 to 1.1% BODT was observed over a period of 5 days. If released into water, diethyl ether is not expected to adsorb to suspended solids and sediment in water based upon the estimated Koc. Volatilization from water surfaces is expected to be an important fate process based upon this compound's Henry's Law constant. Estimated volatilization half-lives from a model river and model lake are 3.1 hours and 3.6 days, respectively. BCFs ranging from 0.9 to 9.1 in carp suggest bioconcentration in aquatic organisms is low. Hydrolysis is not expected to occur due to the lack of hydrolyzable functional groups. Occupational exposure to diethyl ether may occur through inhalation and dermal contact with this compound at workplaces where diethyl ether is produced or used. The general population may be exposed to diethyl ether from consumer products, inhalation of ambient air, and ingestion of contaminated drinking water. (HSDB) Section XIII. Disposal Considerations Waste DisposalRecycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when disposing of the substance. Section XIV. Transport Information DOT ClassificationCLASS 4.3: Dangerous when wet material. CLASS 3: Flammable liquid. PIN Number Proper Shipping Name Organometallic substance, liquid, water-reactive, flammable Packing Group (PG)I DOT Pictograms DANGEROUSWHEN WET 4 Continued on Next Page Phenylmagnesium Iodide (42% in Ethyl Ether, ca. 2mol/L) Section XV. Other Regulatory Information and Pictograms TSCA Chemical InventoryThis product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40 CFR 720.36 (C) for those products not on the inventory list: (EPA) (i) These products are supplied solely for use in research and development by or under the supervision of a technically qualified individual as defined in 40 CFR 720.0 et sec. (ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be supplied on an MSDS sheet. WHMIS ClassificationCLASS B-2: Flammable liquid with a flash point lower than 37.8°C (100°F). CLASS E: Corrosive liquid. (Canada) EINECS Number (EEC) 200-467-2 (Ethyl Ether) EEC Risk Statements R12- Extremely flammable. R14- Reacts violently with water. R19- May form explosive peroxides. R20/21/22- Harmful by inhalation, in contact with skin and if swallowed. R34- Causes burns. SECTION 16 - ADDITIONAL INFORMATION N/A |
| HS Code | 2931900090 |
|---|
|
~% 
16002-63-4 |
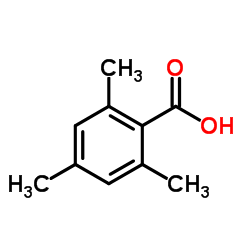
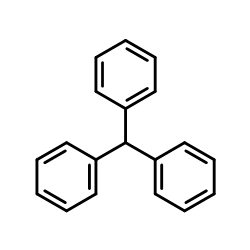
| Literature: Russian Journal of General Chemistry, , vol. 64, # 1.1 p. 32 - 34 Zhurnal Obshchei Khimii, , vol. 64, # 1 p. 35 - 37 |
|
~% 
16002-63-4
Detail
|
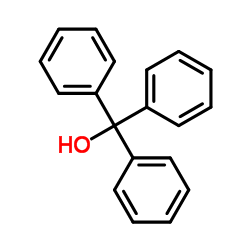
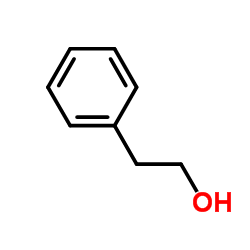
| Literature: Journal of the American Chemical Society, , vol. 61, p. 731,734 |
|
~% 
16002-63-4 |
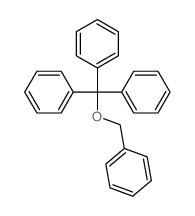
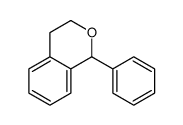
| Literature: Chemische Berichte, , vol. 41, p. 2303 Journal of the Chemical Society, , vol. 93, p. 71 |
| Precursor 4 | |
|---|---|
| DownStream 10 | |
| HS Code | 2931900090 |
|---|---|
| Summary | 2931900090. other organo-inorganic compounds. VAT:17.0%. Tax rebate rate:13.0%. Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward). MFN tariff:6.5%. General tariff:30.0% |

![3,3-Diphenyl-3H-benzo[f]chromene structure](https://image.chemsrc.com/caspic/178/4222-20-2.png)