710-77-0
| Name | 5-ethyl-5-(2-hydroxyethyl)-1,3-diazinane-2,4,6-trione |
|---|---|
| Synonyms |
5-Aethyl-5-(2-hydroxy-aethyl)-barbitursaeure
5-ethyl-5-(2-hydroxyethyl)pyrimidine-2,4,6(1h,3h,5h)-trione 5-Ethyl-5-(2-hydroxyethyl)barbituric acid Barbituric acid,5-ethyl-5-(2-hydroxyethyl) 2,4,6(1H,3H,5H)-Pyrimidinetrione,5-ethyl-5-(2-hydroxyethyl) 5-ethyl-5-(2-hydroxyethyl)-2,4,6(1H,3H,5H)-pyrimidinetrione |
| Molecular Formula | C8H12N2O4 |
|---|---|
| Molecular Weight | 200.19200 |
| Exact Mass | 200.08000 |
| PSA | 95.50000 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
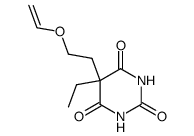
~69% 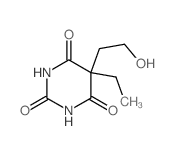
710-77-0 |
| Literature: Lafont; Chastang; Cave; Miocque European Journal of Medicinal Chemistry, 1988 , vol. 23, # 3 p. 283 - 289 |
|
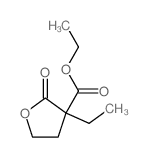
~% 
710-77-0 |
| Literature: Goldschmidt; Wehr Hoppe-Seyler's Zeitschrift fuer Physiologische Chemie, 1957 , vol. 308, p. 9,15 |
|
~% 
710-77-0 |
| Literature: Goldschmidt; Wehr Hoppe-Seyler's Zeitschrift fuer Physiologische Chemie, 1957 , vol. 308, p. 9,15 |
|
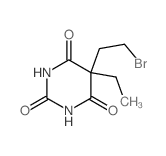
~74% 
710-77-0 |
| Literature: Lafont; Chastang; Cave; Miocque European Journal of Medicinal Chemistry, 1988 , vol. 23, # 3 p. 283 - 289 |
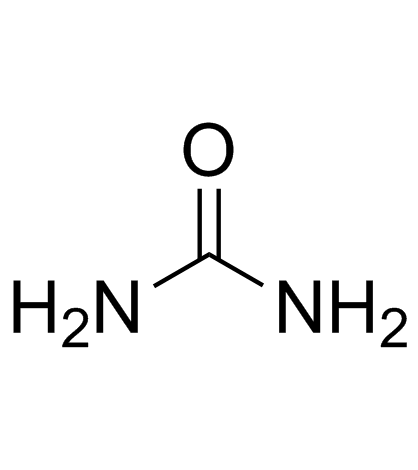
| Precursor 3 | |
|---|---|
| DownStream 1 | |