174022-42-5
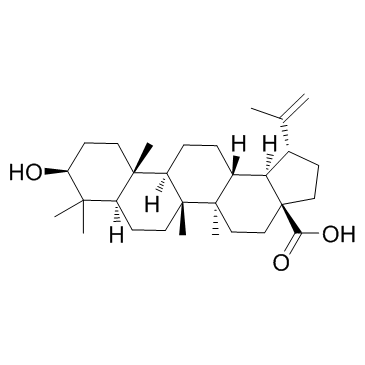
| Name | bevirimat |
|---|---|
| Synonyms |
DSB
UNII-S125DW66N8 3-O-(3',3'-dimethylsuccinyl)betulinic acid YK FH312 YK-FH312 PA-457 BVM (1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-(3-carboxy-3-methylbutanoyl)oxy-5a,5b,8,8,11a-pentamethyl-1-prop-1-en-2-yl-1,2,3,4,5,6,7,7a,9,10,11,11b,12,13,13a,13b-hexadecahydrocyclopenta[a]chrysene-3a-carboxylic acid Bevirimat Butanedioic acid, 2,2-dimethyl-, 4-[(3β)-28-hydroxy-28-oxolup-20(29)-en-3-yl] ester (3β)-3-[(3-Carboxy-3-methylbutanoyl)oxy]lup-20(29)-en-28-oic acid |
| Description | Bevirimat(YK FH312; FH11327; MPC-4326) is an anti-HIV drug derived from a betulinic acid-like compound; is believed to inhibit HIV by a novel mechanism, so-called maturation inhibition.IC50 value:Target: Anti-HIVLike protease inhibitors, bevirimat and other maturation inhibitors interfere with protease processing of newly translated HIV polyprotein precursor, called gag. Bevirimat prevents this viral replication by specifically inhibiting cleavage of the capsid protein (CA) from the SP1 spacer protein. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 662.7±40.0 °C at 760 mmHg |
| Molecular Formula | C36H56O6 |
| Molecular Weight | 584.826 |
| Flash Point | 197.7±20.8 °C |
| Exact Mass | 584.407715 |
| PSA | 100.90000 |
| LogP | 10.15 |
| Vapour Pressure | 0.0±4.3 mmHg at 25°C |
| Index of Refraction | 1.548 |
| Storage condition | -20°C |