2-Formyl-1H-pyrrole
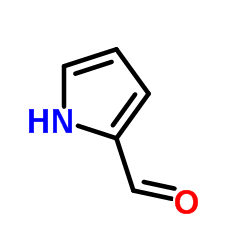
2-Formyl-1H-pyrrole structure
|
Common Name | 2-Formyl-1H-pyrrole | ||
|---|---|---|---|---|
| CAS Number | 1003-29-8 | Molecular Weight | 95.099 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 219.1±13.0 °C at 760 mmHg | |
| Molecular Formula | C5H5NO | Melting Point | 43-46 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 106.7±0.0 °C | |
Use of 2-Formyl-1H-pyrrolePyrrole-2-carboxaldehyde has vibrational and electronic characteristics used to establish the existence of dimeric form in solid phase and monomeric form in solution phase[1]. |
| Name | pyrrole-2-carboxaldehyde |
|---|---|
| Synonym | More Synonyms |
| Description | Pyrrole-2-carboxaldehyde has vibrational and electronic characteristics used to establish the existence of dimeric form in solid phase and monomeric form in solution phase[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 219.1±13.0 °C at 760 mmHg |
| Melting Point | 43-46 °C(lit.) |
| Molecular Formula | C5H5NO |
| Molecular Weight | 95.099 |
| Flash Point | 106.7±0.0 °C |
| Exact Mass | 95.037117 |
| PSA | 32.86000 |
| LogP | 0.64 |
| Vapour Pressure | 0.1±0.4 mmHg at 25°C |
| Index of Refraction | 1.607 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S24/25-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Spectroscopic characterization and biological activity of Zn(II), Cd(II), Sn(II) and Pb(II) complexes with Schiff base derived from pyrrole-2-carboxaldehyde and 2-amino phenol.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 76(3-4) , 376-83, (2010) A new Schiff base 2-aminophenol-pyrrole-2-carboxaldehyde and its Zn(II), Cd(II), Sn(II) and Pb(II) complexes have been synthesized and characterized by various physicochemical studies. Spectral studie... |
|
|
Mutagen formation in the reaction of Maillard browning products, 2-acetylpyrrole and its analogues, with nitrite.
Food Chem. Toxicol. 24(12) , 1303-8, (1986) Three 2-substituted pyrroles (2-acetylpyrrole, pyrrole-2-carboxaldehyde and pyrrole-2-carboxylic acid), which are products of the Maillard browning reaction, were reacted with nitrite in buffer soluti... |
|
|
Suppression of blood lipid concentrations by volatile Maillard reaction products.
Nutrition 24(11-12) , 1159-66, (2008) Although the chemistry of Maillard reaction products (MRPs) in foods has been well studied, few reports on the nutritional characteristics of MRPs in experimental animals and humans have been found. I... |
| 1H-Pyrrole-2-carbaldehyde |
| 1H-Pyrrole-2-carboxaldehyde |
| EINECS 213-705-5 |
| 2-PCA |
| 2-PYRROLYLCARBOXALDEHYDE |
| A-PYRROLALDEHYDE |
| PYRROLE-2-ALDEHYDE |
| 2-Pyrrolecarbaldehyde |
| pyrrole-2-carbaldehyde |
| 2-Pyrrolaldehyde |
| 2-Pyrrolecarboxaldehyde |
| pyrrol-2-carboxaldehyde |
| Pyrrole-2-carboxaldehyde (8CI) |
| formylpyrrole |
| 2-FORMYL-1H-PYRROLE |
| pyrrole-2-carboxaldehyde |
| MFCD00005217 |
| 2-Formylpyrrole |
 CAS#:4513-94-4
CAS#:4513-94-4 CAS#:109-97-7
CAS#:109-97-7 CAS#:68-12-2
CAS#:68-12-2 CAS#:161282-57-1
CAS#:161282-57-1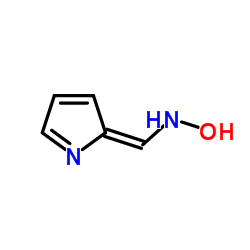 CAS#:32597-34-5
CAS#:32597-34-5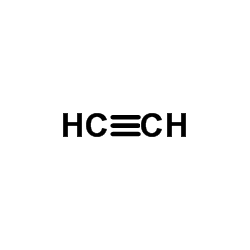 CAS#:74-86-2
CAS#:74-86-2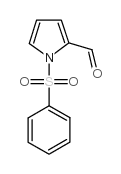 CAS#:86688-93-9
CAS#:86688-93-9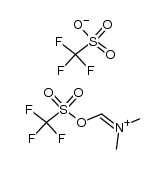 CAS#:132407-64-8
CAS#:132407-64-8 CAS#:104501-02-2
CAS#:104501-02-2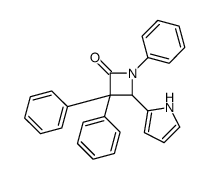 CAS#:105090-44-6
CAS#:105090-44-6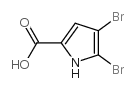 CAS#:34649-21-3
CAS#:34649-21-3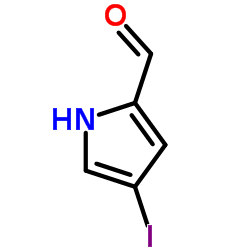 CAS#:33515-62-7
CAS#:33515-62-7![4-Bromopyrrolo[2,1-f][1,2,4]triazine structure](https://image.chemsrc.com/caspic/274/310436-61-4.png) CAS#:310436-61-4
CAS#:310436-61-4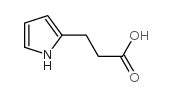 CAS#:408309-29-5
CAS#:408309-29-5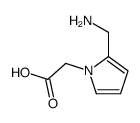 CAS#:145041-27-6
CAS#:145041-27-6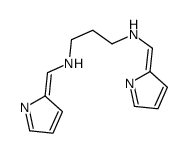 CAS#:14942-62-2
CAS#:14942-62-2
