Zardaverine
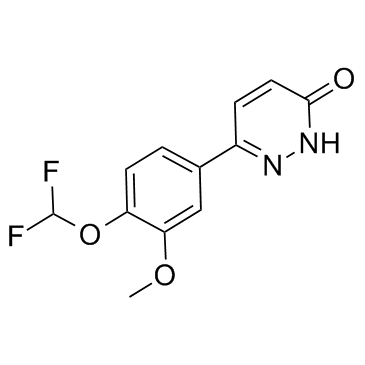
Zardaverine structure
|
Common Name | Zardaverine | ||
|---|---|---|---|---|
| CAS Number | 101975-10-4 | Molecular Weight | 268.216 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C12H10F2N2O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of ZardaverineZardaverine is a newly developed dual-selective PDE3/4 inhibitor with IC50 values of 0.5 uM and 0.8 uM respectively. IC50 value: 0.5 uM (PDE3); 0.8 uM (PDE4)Target: PDE3; PDE4Zardaverine inhibited the cyclic GMP-inhibitable PDE III from human platelets and the rolipram-inhibitable PDE IV from canine trachea and human polymorphonuclear (PMN) cells with IC50-values of 0.58, 0.79 and 0.17 μM, respectively. The pyridazinone derivative affected the calmodulin-stimulated PDE I, the cyclic GMP-stimulated PDE II and the cyclic GMP-specific PDE V only marginally at concentrations up to 100μM. Zardaverine inhibits the ADP-induced aggregation of human platelets with an IC50 of 1.6 μM. This inhibition was synergistically increased by activators of adenylate cyclase such as PGE1 and forskolin. In human PMN cells, Zardaverine inhibited the zymosan-induced superoxide anion generation with an IC50 of 0.40 μM. Again, this effect was increased by activators of adenylate cyclase. Zardaverine acted in synergy with the adenylate cyclase activators prostaglandin E2 and CG 4203, a prostacyclin analog, and super-additive effects of combinations were observed. Zardaverine and dexamethasone prevent bronchial eosinophilia and neutrophilia with similar dosage of 30 microM/kg orally, suggesting that this PDE III/IV inhibitor may be useful for both, bronchorelaxation and reduction of inflammation in asthma therapy. |
| Name | zardaverine |
|---|---|
| Synonym | More Synonyms |
| Description | Zardaverine is a newly developed dual-selective PDE3/4 inhibitor with IC50 values of 0.5 uM and 0.8 uM respectively. IC50 value: 0.5 uM (PDE3); 0.8 uM (PDE4)Target: PDE3; PDE4Zardaverine inhibited the cyclic GMP-inhibitable PDE III from human platelets and the rolipram-inhibitable PDE IV from canine trachea and human polymorphonuclear (PMN) cells with IC50-values of 0.58, 0.79 and 0.17 μM, respectively. The pyridazinone derivative affected the calmodulin-stimulated PDE I, the cyclic GMP-stimulated PDE II and the cyclic GMP-specific PDE V only marginally at concentrations up to 100μM. Zardaverine inhibits the ADP-induced aggregation of human platelets with an IC50 of 1.6 μM. This inhibition was synergistically increased by activators of adenylate cyclase such as PGE1 and forskolin. In human PMN cells, Zardaverine inhibited the zymosan-induced superoxide anion generation with an IC50 of 0.40 μM. Again, this effect was increased by activators of adenylate cyclase. Zardaverine acted in synergy with the adenylate cyclase activators prostaglandin E2 and CG 4203, a prostacyclin analog, and super-additive effects of combinations were observed. Zardaverine and dexamethasone prevent bronchial eosinophilia and neutrophilia with similar dosage of 30 microM/kg orally, suggesting that this PDE III/IV inhibitor may be useful for both, bronchorelaxation and reduction of inflammation in asthma therapy. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C12H10F2N2O3 |
| Molecular Weight | 268.216 |
| Exact Mass | 268.065948 |
| PSA | 64.21000 |
| LogP | 0.90 |
| Index of Refraction | 1.555 |
| Storage condition | 2-8℃ |
|
Section1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE Product identifiers Product name: Zardaverine CAS-No.: 101975-10-4 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Laboratory chemicals, Manufacture of substances Section2. HAZARDS IDENTIFICATION Classification of the substance or mixture Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008. This substance is not classified as dangerous according to Directive 67/548/EEC. Label elements The product does not need to be labelled in accordance with EC directives or respective national laws. Other hazards - none Section3. COMPOSITION/INFORMATION ON INGREDIENTS Substances Synonyms: 6-(4-Difluoromethoxy-3-methoxyphenyl)-3(2H)-pyridazinone Formula: C12H10F2N2O3 Molecular Weight: 268,22 g/mol Section4. FIRST AID MEASURES Description of first aid measures If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. In case of skin contact Wash off with soap and plenty of water. In case of eye contact Flush eyes with water as a precaution. If swallowed Never give anything by mouth to an unconscious person. Rinse mouth with water. Most important symptoms and effects, both acute and delayed To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated. Indication of any immediate medical attention and special treatment needed no data available Section5. FIREFIGHTING MEASURES Extinguishing media Suitable extinguishing media Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide. Special hazards arising from the substance or mixture Carbon oxides, nitrogen oxides (NOx), Hydrogen fluoride Advice for firefighters Wear self contained breathing apparatus for fire fighting if necessary. Further information no data available Section6. ACCIDENTAL RELEASE MEASURES Personal precautions, protective equipment and emergency procedures Avoid dust formation. Avoid breathing vapors, mist or gas. Environmental precautions Do not let product enter drains. Methods and materials for containment and cleaning up Sweep up and shovel. Keep in suitable, closed containers for disposal. Reference to other sections For disposal see section 13. Section7. HANDLING AND STORAGE Precautions for safe handling Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire protection. Conditions for safe storage, including any incompatibilities Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Specific end uses no data available Section8. EXPOSURE CONTROLS/PERSONAL PROTECTION Control parameters Components with workplace control parameters Exposure controls Appropriate engineering controls General industrial hygiene practice. Personal protective equipment Eye/face protection Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU). Skin protection Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it. Body Protection Choose body protection in relation to its type, to the concentration and amount of dangerous substances, and to the specific work-place., The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Respiratory protection Respiratory protection is not required. Where protection from nuisance levels of dusts are desired, use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU). Section9. PHYSICAL AND CHEMICAL PROPERTIES Information on basic physical and chemical properties a) AppearanceForm: solid b) Odourno data available c) Odour Thresholdno data available d) pHno data available e) Melting point/freezingno data available point f) Initial boiling point and no data available boiling range g) Flash pointno data available h) Evaporation rateno data available i) Flammability (solid, gas) no data available j) Upper/lowerno data available flammability or explosive limits k) Vapour pressureno data available l) Vapour densityno data available m) Relative densityno data available n) Water solubilityno data available o) Partition coefficient: n- no data available octanol/water p) Autoignitionno data available temperature q) Decompositionno data available temperature r) Viscosityno data available s) Explosive propertiesno data available t) Oxidizing propertiesno data available Other safety information no data available Section10. STABILITY AND REACTIVITY Reactivity no data available Chemical stability no data available Possibility of hazardous reactions no data available Conditions to avoid no data available Incompatible materials Strong oxidizing agents Hazardous decomposition products Other decomposition products - no data available Section11. TOXICOLOGICAL INFORMATION Information on toxicological effects Acute toxicity no data available Skin corrosion/irritation no data available Serious eye damage/eye irritation no data available Respiratory or skin sensitization no data available Germ cell mutagenicity no data available Carcinogenicity IARC:No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC. Reproductive toxicity no data available Specific target organ toxicity - single exposure no data available Specific target organ toxicity - repeated exposure no data available Aspiration hazard no data available Potential health effects Inhalation May be harmful if inhaled. May cause respiratory tract irritation. IngestionMay be harmful if swallowed. SkinMay be harmful if absorbed through skin. May cause skin irritation. EyesMay cause eye irritation. Signs and Symptoms of Exposure To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated. Additional Information RTECS: Not available Section12. ECOLOGICAL INFORMATION Toxicity no data available Persistence and degradability no data available Bioaccumulative potential no data available Mobility in soil no data available Results of PBT and vPvB assessment no data available Other adverse effects no data available Section13. DISPOSAL CONSIDERATIONS Waste treatment methods Product Offer surplus and non-recyclable solutions to a licensed disposal company. Contaminated packaging Dispose of as unused product. Section14. TRANSPORT INFORMATION UN number ADR/RID: -IMDG: -IATA: - UN proper shipping name ADR/RID: Not dangerous goods IMDG: Not dangerous goods IATA:Not dangerous goods Transport hazard class(es) ADR/RID: -IMDG: -IATA: - Packaging group ADR/RID: -IMDG: -IATA: - Environmental hazards ADR/RID: noIMDG Marine pollutant: noIATA: no Special precautions for user no data available SECTION 15 - REGULATORY INFORMATION N/A SECTION 16 - ADDITIONAL INFORMATION N/A |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
|
~% 
Zardaverine CAS#:101975-10-4 |
| Literature: US4665074 A1, ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
|
Uncovering caffeine's adenosine A2A receptor inverse agonism in experimental parkinsonism.
ACS Chem. Biol. 9(11) , 2496-501, (2014) Caffeine, the most consumed psychoactive substance worldwide, may have beneficial effects on Parkinson's disease (PD) therapy. The mechanism by which caffeine contributes to its antiparkinsonian effec... |
|
|
Dopamine receptors D3 and D5 regulate CD4(+)T-cell activation and differentiation by modulating ERK activation and cAMP production.
J. Neuroimmunol. 284 , 18-29, (2015) Dopamine receptors have been described in T-cells, however their signalling pathways coupled remain unknown. Since cAMP and ERKs play key roles regulating T-cell physiology, we aim to determine whethe... |
|
|
Phenotype-based screening of mechanistically annotated compounds in combination with gene expression and pathway analysis identifies candidate drug targets in a human squamous carcinoma cell model.
J. Biomol. Screen. 11(5) , 457-68, (2006) The squamous cell carcinoma HeLa cell line and an epithelial cell line hTERT-RPE with a nonmalignant phenotype were interrogated for HeLa cell selectivity in response to 1267 annotated compounds repre... |
| 3-[4-(difluoromethoxy)-3-methoxyphenyl]-1H-pyridazin-6-one |
| 3-pyridazinol, 6-[4-(difluoromethoxy)-3-methoxyphenyl]- |
| Zardaverine |
| 3(2H)-Pyridazinone, 6-[4-(difluoromethoxy)-3-methoxyphenyl]- |
| 6-[4-(Difluoromethoxy)-3-methoxyphenyl]-3(2H)-pyridazinone |
| zardaverinum |
| zardaverina |
| 6-[4-(Difluoromethoxy)-3-methoxyphenyl]pyridazin-3(2H)-one |
![1-[4-(Difluoromethoxy)-3-methoxyphenyl]ethan-1-one structure](https://image.chemsrc.com/caspic/475/101975-20-6.png)