Tubeimoside I
Modify Date: 2024-01-02 15:51:45
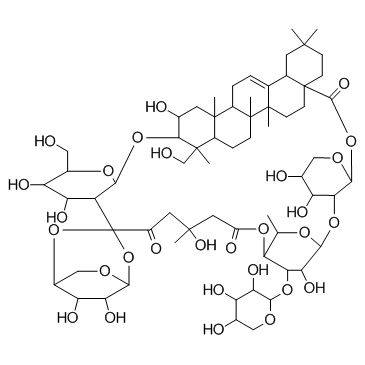
Tubeimoside I structure
|
Common Name | Tubeimoside I | ||
|---|---|---|---|---|
| CAS Number | 102040-03-9 | Molecular Weight | 1319.435 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C63H98O29 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Tubeimoside ITubeimoside I(Lobatoside-H) is an extract from Chinese herbal medicine Bolbostemma paniculatum (MAXIM.) FRANQUET (Cucurbitaceae) has been shown as a potent anti-tumor agent for a variety of human cancers.IC50 value:Target: Anticancer natural compoundin vitro: TBMS I inhibited the proliferation of both HepG2 and L-02 cells in a dose- and time-dependent manner, but HepG2 cells appeared more sensitive to the agent. When exposed to TBMS I for 24, 48 and 72 h, IC50 for HepG2 cells versus L-02 cells were 15.5 vs. 23.1, 11.7 vs. 16.2, 9.2 vs. 13.1 (μM, p<0.01), respectively. TBMS I induced cell shrinkage, nuclear condensation and fragmentation, cell cycle arrest at the G2/M phase, mitochondrial membrane disruption, release of cytochrome c from the mitochondria, activation of caspase 3 and 9, and shifting Bax/Bcl-2 ratio from being anti-apoptotic to pro-apoptotic, all indicative of initiation and progression of apoptosis involving mitochondrial dysfunction [1]. TBMS1-induced molecular events were related to mitochondria-induced intrinsic apoptosis and P21-cyclin B1/cdc2 complex-related G2/M cell cycle arrest [2]. TBMS1 combined with CDDP promoted cell apoptosis, decreased proliferation activity and increased cytosolic Ca2+ levels. Bcl-2 protein expression was down-regulated but Bax was up-regulated. Moreover, GST-π mRNA and protein expression were decreased. TBMS1 reduced the resistance of the cells to CDDP-induced cytotoxicity [4]. Treatment with TBMS1 resulted in dose- and time-dependent inhibition of proliferation, led to arrest in phase G2/M of the cell cycle and increased the levels of intracellular Ca2?. Furthermore, TBMS1 up-regulated the levels of the glucose-regulated protein 78/immunoglobuin heavy chain binding protein (GRP78/Bip), C/EBP homologous protein (CHOP), Bax, and cleaved caspase-3 and down-regulated the levels of Bcl-2 [5].in vivo: TBMS1 significantly inhibited the production of the pro-inflammatory cytokines, TNF-α, IL-6 and IL-1β in vitro and in vivo. Pretreatment with TBMS1 markedly attenuated the development of pulmonary edema, histological severities and inflammatory cells infiltration in mice with ALI [3]. |
| Name | Tubeimoside A |
|---|---|
| Synonym | More Synonyms |
| Description | Tubeimoside I(Lobatoside-H) is an extract from Chinese herbal medicine Bolbostemma paniculatum (MAXIM.) FRANQUET (Cucurbitaceae) has been shown as a potent anti-tumor agent for a variety of human cancers.IC50 value:Target: Anticancer natural compoundin vitro: TBMS I inhibited the proliferation of both HepG2 and L-02 cells in a dose- and time-dependent manner, but HepG2 cells appeared more sensitive to the agent. When exposed to TBMS I for 24, 48 and 72 h, IC50 for HepG2 cells versus L-02 cells were 15.5 vs. 23.1, 11.7 vs. 16.2, 9.2 vs. 13.1 (μM, p<0.01), respectively. TBMS I induced cell shrinkage, nuclear condensation and fragmentation, cell cycle arrest at the G2/M phase, mitochondrial membrane disruption, release of cytochrome c from the mitochondria, activation of caspase 3 and 9, and shifting Bax/Bcl-2 ratio from being anti-apoptotic to pro-apoptotic, all indicative of initiation and progression of apoptosis involving mitochondrial dysfunction [1]. TBMS1-induced molecular events were related to mitochondria-induced intrinsic apoptosis and P21-cyclin B1/cdc2 complex-related G2/M cell cycle arrest [2]. TBMS1 combined with CDDP promoted cell apoptosis, decreased proliferation activity and increased cytosolic Ca2+ levels. Bcl-2 protein expression was down-regulated but Bax was up-regulated. Moreover, GST-π mRNA and protein expression were decreased. TBMS1 reduced the resistance of the cells to CDDP-induced cytotoxicity [4]. Treatment with TBMS1 resulted in dose- and time-dependent inhibition of proliferation, led to arrest in phase G2/M of the cell cycle and increased the levels of intracellular Ca2?. Furthermore, TBMS1 up-regulated the levels of the glucose-regulated protein 78/immunoglobuin heavy chain binding protein (GRP78/Bip), C/EBP homologous protein (CHOP), Bax, and cleaved caspase-3 and down-regulated the levels of Bcl-2 [5].in vivo: TBMS1 significantly inhibited the production of the pro-inflammatory cytokines, TNF-α, IL-6 and IL-1β in vitro and in vivo. Pretreatment with TBMS1 markedly attenuated the development of pulmonary edema, histological severities and inflammatory cells infiltration in mice with ALI [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Molecular Formula | C63H98O29 |
| Molecular Weight | 1319.435 |
| Exact Mass | 1318.619385 |
| PSA | 445.19000 |
| LogP | 6.38 |
| Index of Refraction | 1.637 |
| Hazard Codes | Xi |
|---|
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| TUBEIMOSIDE 1 |
| β-D-Xylopyranosyl-(1->3)-6-deoxy-α-L-mannopyranosyl-(1->2)-1-O-{[(2R,3S,4S,4aR,5aS,8S,9S,9aR,17aR,17bR,19aR,19bS,21aS,25aS,27aR,27bR,29S,29aR,30aR)-3,4,8,9,13,29-hexahydroxy-2-(hydroxymethyl)-1 3,17a,19a,19b,24,24,27b-heptamethyl-11,15-dioxo-3,4,4a,7,8,9,9a,12,13,14,15,17a,18,19,19a,19b,20,21,22,23,24,25,25a,27,27a,27b,28,29,29a,30a-triacontahydro-2H,5aH,11H,17H-piceno[3,4-h]dipyrano[3,2-b:3 ;',2'-e][1,4,7,11]tetraoxacyclohexadecin-21a( |
| TubeimosideI |
| TUBELMOSIDEA |
| TUBEMOSIDE A |
| TUBEIMOSIDE I (RG) |
| (1S,4S,7S,8S,9R,11S,13S,14S,18S,22S,25S,27R,28S,29S,30R,32R,34R,35S,37R,38R,41R,42R,46S,53S,54R,55R,56R,57S,58R)-7,8,18,28,29,35,55,56,58-Nonahydroxy-30,54-bis(hydroxymethyl)-13,18,37,41,48,48,53,54-o ;ctamethyl-2,16,20-trioxo-3,5,10,12,15,21,24,26,31,33-decaoxadecacyclo[39.9.3.2.2.1.0.0.0.0.0]octapentacont-44-en-57-yl β-D-xylopyranoside |
| Olean-12-en-28-oicacid,3-[[2-O-[4-O-(4-carboxy-3 |
| α-L-Arabinopyranose, O-β-D-xylopyranosyl-(1->3)-O-6-deoxy-α-L-mannopyranosyl-(1->2)-1-O-[[(2R,3S,4S,4aR,5aS,8S,9S,9aR,17aR,17bR,19aR,19bS,21aS,25aS,27aR,27bR,29S,29aR,30aR)-3,4,4a,5a,8,9,9a ,12,13,14,15,17a,18,19,19a,19b,20,21,22,23,24,25,25a,27,27a,27b,28,29,29a,30a-triacontahydro-3,4,8,9,13,29-hexahydroxy-2-(hydroxymethyl)-13,17a,19a,19b,24,24,27b-heptamethyl-11,15-dioxo-2H,7H,11H,17H- piceno[3,4-h]dipyrano[3,2-b:3',2'-e][1,4,7,1 |
| Lobatoside H |
| Tubeimoside I |
 CAS#:503-49-1
CAS#:503-49-1
