VX-222 (VCH-222, Lomibuvir)
Modify Date: 2024-01-11 15:40:53
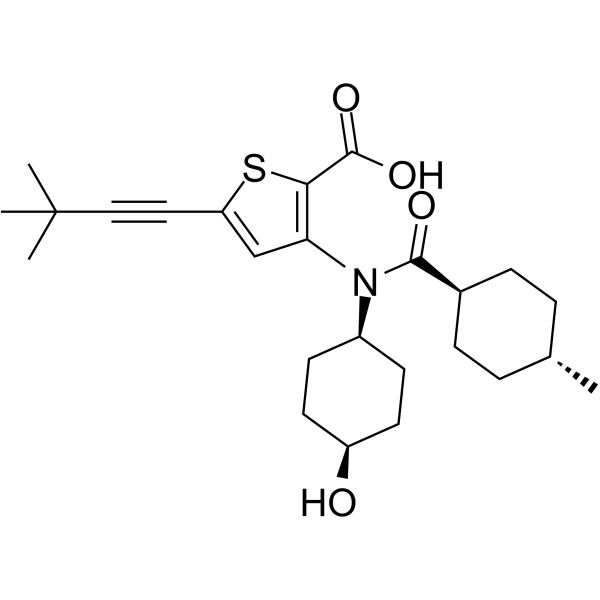
VX-222 (VCH-222, Lomibuvir) structure
|
Common Name | VX-222 (VCH-222, Lomibuvir) | ||
|---|---|---|---|---|
| CAS Number | 1026785-59-0 | Molecular Weight | 445.615 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 640.5±55.0 °C at 760 mmHg | |
| Molecular Formula | C25H35NO4S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 341.2±31.5 °C | |
Use of VX-222 (VCH-222, Lomibuvir)VX-222 (VCH-222) is a novel, potent and selective inhibitor of HCV polymerase with IC50 of 0.94-1.2 μM, 15.3-fold less effective for mutant M423T, and 108-fold less effective for mutant I482L.IC50 Value: 0.94 μM (HCV NS5B 1a); 1.2 μM (HCV NS5B 1b)Target: HCVVX-222 is a small molecule non-nucleoside inhibitor of HCV NS5B polymerase that is being investigated for the treatment of hepatitis C virus infection. VX-222 exhibits non-competitive and selective inhibition in HCV NS5B of genotype 1a and 1b, with IC50 of 0.94 and 1.2 μM, respectively. VX-222 selectively inhibits the replication of subgenomic HCV genotype 1a and 1b with an EC50 of 22.3 and 11.2 nM, respectively. [1] Similarly, a recent study shows that VX-222 inhibits the 1b/Con1 HCV subgenomic replicon, with an EC50 of 5 nM. In rats and dogs, VCH-222 displays fine pharmacokinetic pro le, including low total body clearance and excellent oral bioavailability (greater than 30%) and good ADME properties. VCH-222 is biotransformed by several enzymes (CYP1A1, 2A6, 2B6, 2C8, CYP 3A4, UGT1A3) and is predicted to be actively transported in liver and excreted mainly intact in bile or as glucuronide adducts. |
| Name | vx-222 |
|---|---|
| Synonym | More Synonyms |
| Description | VX-222 (VCH-222) is a novel, potent and selective inhibitor of HCV polymerase with IC50 of 0.94-1.2 μM, 15.3-fold less effective for mutant M423T, and 108-fold less effective for mutant I482L.IC50 Value: 0.94 μM (HCV NS5B 1a); 1.2 μM (HCV NS5B 1b)Target: HCVVX-222 is a small molecule non-nucleoside inhibitor of HCV NS5B polymerase that is being investigated for the treatment of hepatitis C virus infection. VX-222 exhibits non-competitive and selective inhibition in HCV NS5B of genotype 1a and 1b, with IC50 of 0.94 and 1.2 μM, respectively. VX-222 selectively inhibits the replication of subgenomic HCV genotype 1a and 1b with an EC50 of 22.3 and 11.2 nM, respectively. [1] Similarly, a recent study shows that VX-222 inhibits the 1b/Con1 HCV subgenomic replicon, with an EC50 of 5 nM. In rats and dogs, VCH-222 displays fine pharmacokinetic pro le, including low total body clearance and excellent oral bioavailability (greater than 30%) and good ADME properties. VCH-222 is biotransformed by several enzymes (CYP1A1, 2A6, 2B6, 2C8, CYP 3A4, UGT1A3) and is predicted to be actively transported in liver and excreted mainly intact in bile or as glucuronide adducts. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 640.5±55.0 °C at 760 mmHg |
| Molecular Formula | C25H35NO4S |
| Molecular Weight | 445.615 |
| Flash Point | 341.2±31.5 °C |
| Exact Mass | 445.228668 |
| PSA | 106.08000 |
| LogP | 5.15 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.589 |
| Storage condition | -20℃ |
| 5-(3,3-Dimethyl-1-butyn-1-yl)-3-{(cis-4-hydroxycyclohexyl)[(trans-4-methylcyclohexyl)carbonyl]amino}-2-thiophenecarboxylic acid |
| 2-Thiophenecarboxylic acid, 5-(3,3-dimethyl-1-butyn-1-yl)-3-[(cis-4-hydroxycyclohexyl)[(trans-4-methylcyclohexyl)carbonyl]amino]- |
| vx-222 vx222 |
| VX222 |