4-Nitrophenyl α-D-mannopyranoside

4-Nitrophenyl α-D-mannopyranoside structure
|
Common Name | 4-Nitrophenyl α-D-mannopyranoside | ||
|---|---|---|---|---|
| CAS Number | 10357-27-4 | Molecular Weight | 301.249 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 582.2±50.0 °C at 760 mmHg | |
| Molecular Formula | C12H15NO8 | Melting Point | 179-181ºC | |
| MSDS | USA | Flash Point | 305.9±30.1 °C | |
Use of 4-Nitrophenyl α-D-mannopyranosidep-Nitrophenyl α-D-mannopyranoside is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 4-Nitrophenyl a-D-mannopyranoside |
|---|---|
| Synonym | More Synonyms |
| Description | p-Nitrophenyl α-D-mannopyranoside is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 582.2±50.0 °C at 760 mmHg |
| Melting Point | 179-181ºC |
| Molecular Formula | C12H15NO8 |
| Molecular Weight | 301.249 |
| Flash Point | 305.9±30.1 °C |
| Exact Mass | 301.079773 |
| PSA | 145.20000 |
| LogP | -0.55 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.648 |
| Storage condition | 2-8°C |
| Water Solubility | DMF: 50 mg/mL, clear, very faintly yellow |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 24/25-36-26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2932999099 |
|
~94% 
4-Nitrophenyl α... CAS#:10357-27-4 |
| Literature: Scott, Ian L.; Market, Robert V.; DeOrazio, Russell J.; Meckler, Harold; Kogan, Timothy P. Carbohydrate Research, 1999 , vol. 317, # 1-4 p. 210 - 216 |
|
~% 
4-Nitrophenyl α... CAS#:10357-27-4 |
| Literature: Australian Journal of Chemistry, , vol. 8, p. 403,407 Australian Journal of Chemistry, , vol. 7, p. 202,204 |
|
~% 
4-Nitrophenyl α... CAS#:10357-27-4 |
| Literature: Chemical Communications, , # 3 p. 229 - 230 |
|
~% 
4-Nitrophenyl α... CAS#:10357-27-4 |
| Literature: Chemical Communications, , # 3 p. 229 - 230 |
|
~% 
4-Nitrophenyl α... CAS#:10357-27-4 |
| Literature: Carbohydrate Research, , vol. 317, # 1-4 p. 210 - 216 |
| Precursor 5 | |
|---|---|
| DownStream 7 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Synthesis of glycopeptide dendrimers, dimerization and affinity for Concanavalin A.
Bioorg. Med. Chem. 19 , 2879-87, (2011) We described herein the synthesis of second generation glycopeptide dendrimers G2a-g presenting variable amino acids placed internally into the multivalent scaffold. The effect of such structural modu... |
|
|
ABC transporter A3 facilitates lysosomal sequestration of imatinib and modulates susceptibility of chronic myeloid leukemia cell lines to this drug.
Haematologica 94 , 1528-36, (2009) Inhibition of BCR-ABL tyrosine kinase activity has evolved as a mainstay of therapy for patients with chronic myeloid leukemia. However, a fraction of leukemic cells persists under targeted therapy an... |
|
|
Mechanistic insights into a Ca2+-dependent family of alpha-mannosidases in a human gut symbiont.
Nat. Chem. Biol. 6 , 125-32, (2010) Colonic bacteria, exemplified by Bacteroides thetaiotaomicron, play a key role in maintaining human health by harnessing large families of glycoside hydrolases (GHs) to exploit dietary polysaccharides... |
| MFCD00066002 |
| p-nitrophenyl mannoside |
| 4-Nitrophenyl α-D-mannopyranoside |
| 4-NITROPHENYL-A-D-MANNOSIDE |
| EINECS 233-776-6 |
| MAN1-A-PNP |
| α-D-Mannopyranoside, 4-nitrophenyl |
| p-Nitrophenyl Alpha-D-Mannopyranoside |
| 4-Nitrophenyl α-D-Mannopyranoside [Substrate |
| 4-Nitrophenyla-D-mannopyranoside |
| 4-Nitrophenyl-alpha-D-Mannopyranoside |
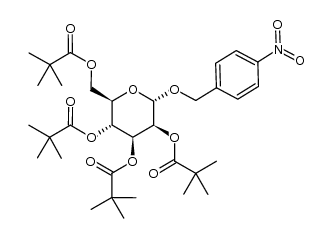
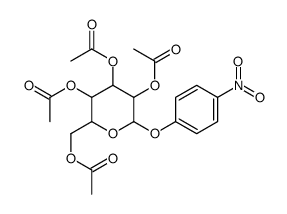

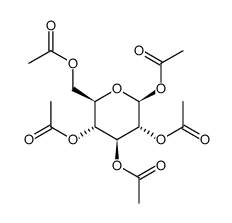
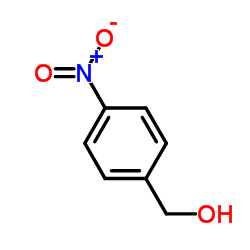
 CAS#:3150-24-1
CAS#:3150-24-1![alpha-d-man-[1->2]-alpha-d-man-1->ome structure](https://image.chemsrc.com/caspic/211/59571-75-4.png) CAS#:59571-75-4
CAS#:59571-75-4 CAS#:78962-39-7
CAS#:78962-39-7 CAS#:34213-86-0
CAS#:34213-86-0 CAS#:3458-28-4
CAS#:3458-28-4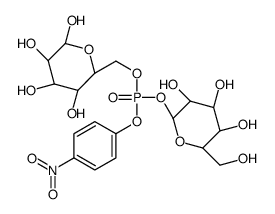 CAS#:101455-34-9
CAS#:101455-34-9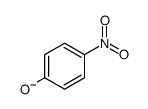 CAS#:14609-74-6
CAS#:14609-74-6