Tacrolimus

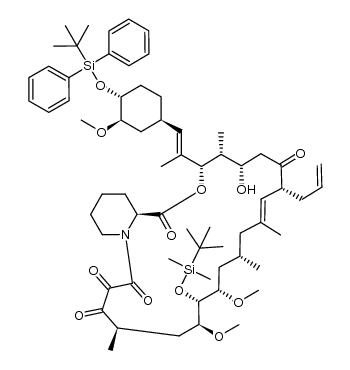
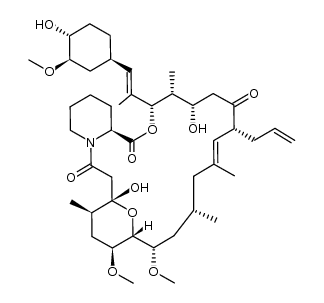
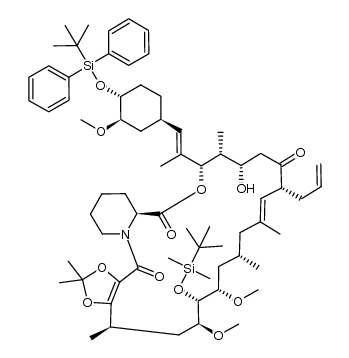
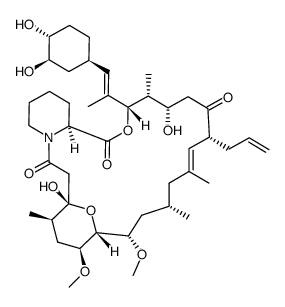
Tacrolimus structure
|
Common Name | Tacrolimus | ||
|---|---|---|---|---|
| CAS Number | 104987-11-3 | Molecular Weight | 804.018 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 871.7±75.0 °C at 760 mmHg | |
| Molecular Formula | C44H69NO12 | Melting Point | 113-115°C | |
| MSDS | N/A | Flash Point | 481.0±37.1 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
Use of TacrolimusTacrolimus is a macrocyclic lactone with potent immunosuppressive properties. Tacrolimus binds to FK506 binding protein (FKBP) to form a complex and inhibits calcineurin phosphatase. |
| Name | FK506 |
|---|---|
| Synonym | More Synonyms |
| Description | Tacrolimus is a macrocyclic lactone with potent immunosuppressive properties. Tacrolimus binds to FK506 binding protein (FKBP) to form a complex and inhibits calcineurin phosphatase. |
|---|---|
| Related Catalog | |
| Target |
PP2B (calcineurin phosphatase)[1] Autophagy inducer[2] |
| In Vitro | Tacrolimus (FK506) inhibits calcium-dependent events, such as IL-2 gene transcription, NO synthase activation, cell degranulation, and apoptosis. Tacrolimus also potentiates the actions of glucocorticoids and progesterone by binding to FKBPs contained within the hormone receptor complex, preventing degradation. The agent may enhance expression of the TGFβ-1 gene in a fashion analogous to that demonstrated for CsA. T cell proliferation in response to ligation of the T cell receptor is inhibited by Tacrolimus[1]. Treatment with a low concentration of Tacrolimus (FK506,10 μg/L) does not significantly affect the proliferation of MH3924A cells (P=0.135). Upon treatment with higher concentrations of Tacrolimus (100-1,000 μg/L), the proliferation of MH3924A cells is significantly enhanced (P<0.01). Treatment with AMD3100 at any concentration (10, 50 or 100 μg/L), has no obvious effect on MH3924A cell proliferation (P>0.05). However, when different concentrations of AMD3100 are combined with 100 μg/L Tacrolimus, the in vitro proliferation of MH3924A cells is increased (P<0.01)[3]. |
| In Vivo | The therapeutic effect of Tacrolimus is investigated on progression and perpetuation of colitis by administering Tacrolimus to Dextran sulfate sodium (DSS)-treated mice from Days 10 to 16 or to 23. At Days 17 and 24, colon length is significantly shortened, and colon weight is significantly higher in DSS-treated control animals than in normal animals. In addition, colon weight per unit length in the control group is more than twice that in the normal group. While both 7 and 14 d treatment with Tacrolimus significantly suppresses increases in colon weight per unit length in DSS-treated animals compared with the control group, this treatment does not actually restore the colon shortening. In addition, this inhibitory effect of Tacrolimus on increases in colon weight per unit length is more pronounced with 14-d than 7-d treatment, as shown by the inhibitory percentages (59% vs. 28%)[4]. |
| Cell Assay | Tumor cell proliferation is determined by the MTT assay. Briefly, after MH3924A cells have reached the logarithmic growth phase, a 0.2-mL cell suspension at 1×104 cells/mL is added into each well of a 96-well plate and cultured in DMEM with 10% FBS, 10 μg/L vascular endothelial growth factor and 0.1 g/L heparin for 24 h. When adherent growth is established, different concentrations of Tacrolimus (10, 100 and 1,000 μg/L) , AMD3100 (10, 50 and 100 μg/L) and Tacrolimus (0 and 100 μg/L)+AMD3100 (0, 10, 50 and 100 μg/L) are added into the plates. Untreated cells cultured in medium alone are used as controls. After culturing for 48 h, 10 μL MTT (5 g/L) are added, and each well is incubated for 6 h; next, 150 μL/well DMSO are added, followed by measurements of the absorbance at 570 mm on a spectrophotometer reader. Each well is measured three times, and each sample is assayed in triplicate[3]. |
| Animal Admin | Mice[4] Six-week-old male C57BL/6J mice are maintained in a temperature- and humidity-controlled room with a 12-h light-dark cycle. For the multiple dosing study, colitic mice (n=10) are orally administered Tacrolimus at 30 mg/kg for 7 d (Days 10 to 16) or 14 d (Days 10 to 23). Control (n=10) and normal groups (n=5) are administered placebo using the same regimen. Tacrolimus or placebo is administered at 10 mL/kg. Mice are euthanized by CO2 inhalation on the day following the final dosing. For the single dosing study, colitic mice are orally administered Tacrolimus at 30 mg/kg or placebo (n=8) once on Day 7, 10, 17, or 24. Normal mice (n=4) are administered placebo using the same regimen. Mice are euthanized by CO2 inhalation eight hours after dosing[4]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 871.7±75.0 °C at 760 mmHg |
| Melting Point | 113-115°C |
| Molecular Formula | C44H69NO12 |
| Molecular Weight | 804.018 |
| Flash Point | 481.0±37.1 °C |
| Exact Mass | 803.481995 |
| PSA | 178.36000 |
| LogP | 3.96 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.549 |
| Storage condition | −20°C |
| Water Solubility | DMSO: >3 mg/mL |
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302 + H312 + H332-H319 |
| Precautionary Statements | P210-P280-P305 + P351 + P338 |
| Hazard Codes | Xi: Irritant;T: Toxic; |
| Risk Phrases | R25;R36/37/38 |
| Safety Phrases | 45-36-26 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | KD4201200 |
| Hazard Class | 6.1 |
|
Unique drug screening approach for prion diseases identifies tacrolimus and astemizole as antiprion agents.
Proc. Natl. Acad. Sci. U. S. A. 110(17) , 7044-9, (2013) Prion diseases such as Creutzfeldt-Jakob disease (CJD) are incurable and rapidly fatal neurodegenerative diseases. Because prion protein (PrP) is necessary for prion replication but dispensable for th... |
|
|
Predictive factors of allosensitization after immunosuppressant withdrawal in recipients of long-term cultured islet cell grafts.
Transplantation 96(2) , 162-9, (2013) Islet transplantation has been reported to induce allosensitization in the majority of type 1 diabetic recipients of fresh or shortly incubated islet grafts prepared from one to three donors.We examin... |
|
|
Effects of rapamycin and tacrolimus on mature endothelial cells and endothelial progenitor cells.
J. Pak. Med. Assoc. 62(8) , 822-5, (2012) To investigate the effects of the potent immunosuppressive agents tacrolimus and rapamycin on the number of circulating mature endothelial cells and circulating endothelial progenitor cells in an expe... |
| Advagraf |
| (-)-Tacrolimus |
| Sunitinib---free base |
| (-)-FK 506 |
| Protopic |
| (1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)-17-Allyl-1,14-dihydroxy-12-{(1E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-propen-2-yl}-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatr ;icyclo[22.3.1.0]octacos-18-ene-2,3,10,16-tetrone |
| (1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)-17-Allyl-1,14-dihydroxy-12-{(1E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-propen-2-yl}-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.0]octacos-18-ene-2,3,10,16-tetrone |
| Sutent-d4 |
| (1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)-17-Allyl-1,14-dihydroxy-12-{(1E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl}-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.0]octacos-18-ene-2,3,10,16-tetrone |
| MFCD00869853 |
| Tacrolimus |
| Suniti |
| FK-506 |
| 15,19-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone, 5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-[(E)-2-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(2-propenyl)-, (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)- |
| (1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)-1,14-dihydroxy-12-{(E)-2-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethenyl}-23,25-dimethoxy-13,19,21,27-tetramethyl-17-prop-2-en-1-yl-11,28-dioxa-4-azatricyclo[22.3.1.0]octacos-18-ene-2,3,10,16-tetrone |
| (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-5,19-dihydroxy-3-{(1E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl}-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(prop-2-en-1-yl)-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-3H-15,19-epoxypyrido[2,1-c][1,4]oxazacyclotricosine-1,7,20,21(4H,23H)-tetrone |
| Prograf |
| 15,19-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone, 5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-[(E)-2-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(2-propen-1-yl)-, (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)- |
| SUNITINIB-D4 |
| SUNITINIB BASE |
| SUNITINIB MALEATE |

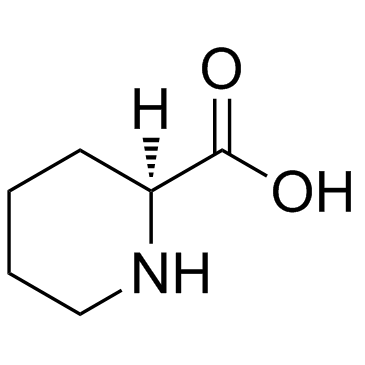 CAS#:3105-95-1
CAS#:3105-95-1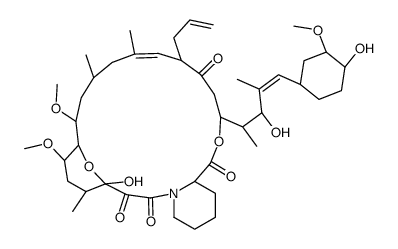 CAS#:134590-88-8
CAS#:134590-88-8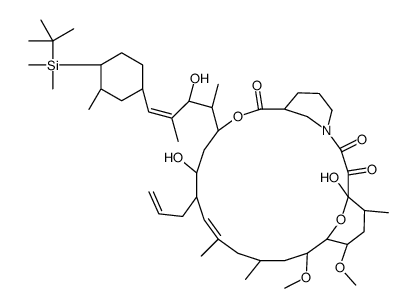 CAS#:134556-79-9
CAS#:134556-79-9
