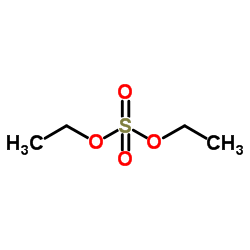
5-Ethylphenazin-5-ium ethyl sulfate
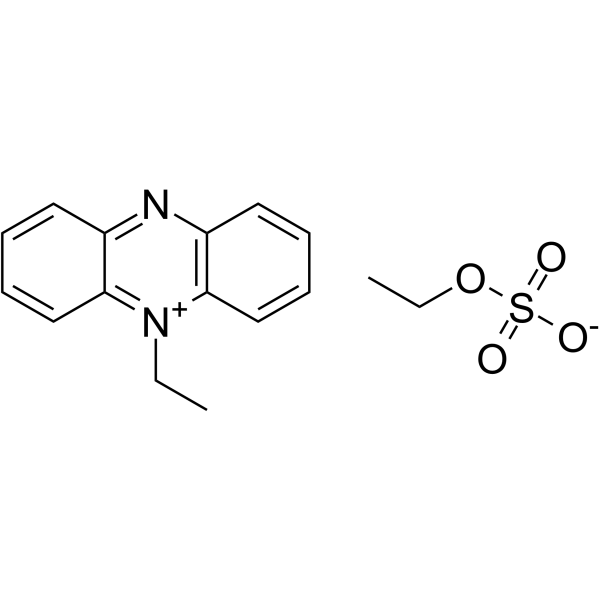
5-Ethylphenazin-5-ium ethyl sulfate structure
|
Common Name | 5-Ethylphenazin-5-ium ethyl sulfate | ||
|---|---|---|---|---|
| CAS Number | 10510-77-7 | Molecular Weight | 334.390 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C16H18N2O4S | Melting Point | 190℃ | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 5-Ethylphenazin-5-ium ethyl sulfatePhenazine ethosulfate is a cationic dye (Ex=390 nm, Em=530 nm) and an electron acceptor that can be used in dye-linked enzyme assays. Phenazine ethosulfate is an intermediate for detecting nitric oxide reducatase (Nors) activity with the presence of ascorbic acid[1][2][3]. |
| Name | Phenazine ethosulfate |
|---|---|
| Synonym | More Synonyms |
| Description | Phenazine ethosulfate is a cationic dye (Ex=390 nm, Em=530 nm) and an electron acceptor that can be used in dye-linked enzyme assays. Phenazine ethosulfate is an intermediate for detecting nitric oxide reducatase (Nors) activity with the presence of ascorbic acid[1][2][3]. |
|---|---|
| Related Catalog | |
| References |
| Melting Point | 190℃ |
|---|---|
| Molecular Formula | C16H18N2O4S |
| Molecular Weight | 334.390 |
| Exact Mass | 334.098724 |
| PSA | 91.58000 |
| LogP | 3.25930 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 36/37/38 |
| Safety Phrases | S26;S36 |
| RIDADR | NONH for all modes of transport |
| RTECS | SG1635000 |
| HS Code | 2933990090 |
|
~% 
5-Ethylphenazin... CAS#:10510-77-7 |
| Literature: Journal of the Chemical Society, , p. 1704,1710 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Organophosphate pesticides-induced changes in the redox status of rat tissues and protective effects of antioxidant vitamins.
Environ. Toxicol. 30(4) , 472-82, (2015) Organophosphates (OPs) pesticides are among the most toxic synthetic chemicals purposefully added in the environment. The common use of OP insecticides in public health and agriculture results in an e... |
|
|
Effects of indoleamine 2,3-dioxygenase inhibitor in non-Hodgkin lymphoma model mice.
Int. J. Hematol. 102 , 327-34, (2015) Indoleamine 2,3-dioxygenase (IDO) catalyzes the rate-limiting step in the metabolism of tryptophan along the kynurenine pathway. In tumors, increased IDO activity inhibits proliferation and induces ap... |
|
|
The anti-lymphoma activity of APO866, an inhibitor of nicotinamide adenine dinucleotide biosynthesis, is potentialized when used in combination with anti-CD20 antibody.
Leuk. Lymphoma 55(9) , 2141-50, (2014) APO866 is an inhibitor of nicotinamide adenine dinucleotide (NAD) biosynthesis that exhibits potent anti-lymphoma activity. Rituximab (RTX), an anti-CD20 antibody, kills lymphoma cells by direct apopt... |
| EINECS 234-044-9 |
| MFCD00050286 |
| 5-Ethylphenazin-5-ium ethyl sulfate |
| 5-ethylphenazin-5-ium,ethyl sulfate |