Citronellal
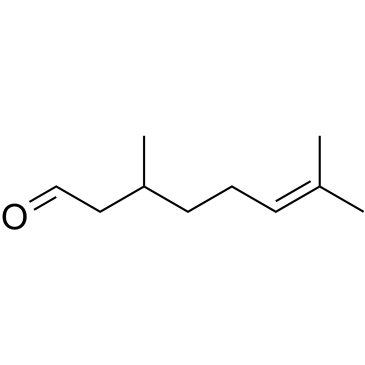
Citronellal structure
|
Common Name | Citronellal | ||
|---|---|---|---|---|
| CAS Number | 106-23-0 | Molecular Weight | 154.249 | |
| Density | 0.8±0.1 g/cm3 | Boiling Point | 208.4±9.0 °C at 760 mmHg | |
| Molecular Formula | C10H18O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 75.6±0.0 °C | |
| Symbol |


GHS07, GHS09 |
Signal Word | Warning | |
Use of CitronellalCitronellal is a monoterpenea from the essential oils in various aromatic species of plants, with depressant, hypnotic, and antinociceptive properties. Citronellal attenuates mechanical nociception, mediated in part by the NO-cGMP-ATP-sensitive K⁺ channel pathway[1][2]. |
| Name | citronellal |
|---|---|
| Synonym | More Synonyms |
| Description | Citronellal is a monoterpenea from the essential oils in various aromatic species of plants, with depressant, hypnotic, and antinociceptive properties. Citronellal attenuates mechanical nociception, mediated in part by the NO-cGMP-ATP-sensitive K⁺ channel pathway[1][2]. |
|---|---|
| Related Catalog | |
| References |
[1]. Melo MS, et al. Antinociceptive effect of citronellal in mice. Pharm Biol. 2010 Apr;48(4):411-6. |
| Density | 0.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 208.4±9.0 °C at 760 mmHg |
| Molecular Formula | C10H18O |
| Molecular Weight | 154.249 |
| Flash Point | 75.6±0.0 °C |
| Exact Mass | 154.135757 |
| PSA | 17.07000 |
| LogP | 3.48 |
| Vapour Pressure | 0.2±0.4 mmHg at 25°C |
| Index of Refraction | 1.437 |
| Storage condition | 2-8°C |
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H317-H319-H335-H411 |
| Precautionary Statements | P273-P280-P333 + P313-P337 + P313-P391 |
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S36/37-S61-S37/39-S26-S36 |
| RIDADR | UN 3082 9/PG 3 |
| WGK Germany | 3 |
| RTECS | RH2140000 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
|
Circuit formation and function in the olfactory bulb of mice with reduced spontaneous afferent activity.
J. Neurosci. 35(1) , 146-60, (2015) The type of neuronal activity required for circuit development is a matter of significant debate. We addressed this issue by analyzing the topographic organization of the olfactory bulb in transgenic ... |
|
|
The Mosquito Repellent Citronellal Directly Potentiates Drosophila TRPA1, Facilitating Feeding Suppression.
Mol. Cells 38 , 911-7, (2015) Citronellal, a well-known plant-derived mosquito repellent, was previously reported to repel Drosophila melanogaster via olfactory pathways involving but not directly activating Transient Receptor Pot... |
|
|
Electrophysiological and behavioral characterization of bioactive compounds of the Thymus vulgaris, Cymbopogon winterianus, Cuminum cyminum and Cinnamomum zeylanicum essential oils against Anopheles gambiae and prospects for their use as bednet treatments.
Parasit. Vectors 8 , 316, (2015) Laboratory and field studies showed that repellent, irritant and toxic actions of common public health insecticides reduce human-vector contact and thereby interrupt disease transmission. One of the m... |
| Citronellal |
| 3,7-dimethyloct-6-en-1-al |
| CITRONELLAL 80 |
| Citronellel |
| d-rhodinal |
| β-Citronellal |
| ALPHA-CITRONELLAL |
| 6-Octenal, 3,7-dimethyl- |
| RHODINAL |
| 3,7-dimethyloct-6-enal |
| 3,7-Dimethyl-6-octenal |
| b-Citronellal |
| UNII:QB99VZZ7GZ |
| (±)-3,7-Dimethyl-6-octenal |
| EINECS 203-376-6 |
| Levo-citronellal |
| dl-Citronellal |
| (±)-citronellal |
| MFCD00038090 |
| Citronella |
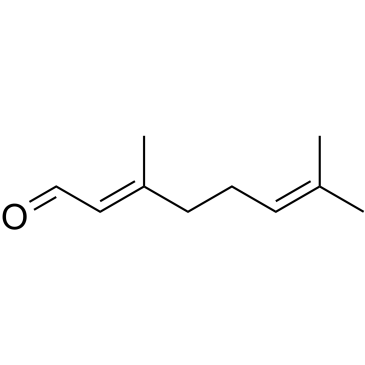 CAS#:5392-40-5
CAS#:5392-40-5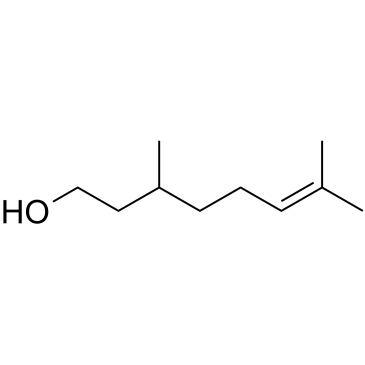 CAS#:106-22-9
CAS#:106-22-9 CAS#:106-26-3
CAS#:106-26-3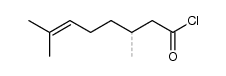 CAS#:36392-06-0
CAS#:36392-06-0 CAS#:56841-52-2
CAS#:56841-52-2 CAS#:106-25-2
CAS#:106-25-2 CAS#:67-63-0
CAS#:67-63-0 CAS#:141-27-5
CAS#:141-27-5 CAS#:502-47-6
CAS#:502-47-6 CAS#:71504-21-7
CAS#:71504-21-7 CAS#:51566-62-2
CAS#:51566-62-2 CAS#:19550-54-0
CAS#:19550-54-0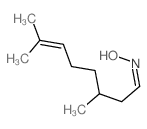 CAS#:22457-25-6
CAS#:22457-25-6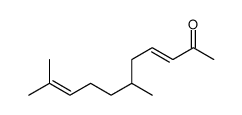 CAS#:19870-49-6
CAS#:19870-49-6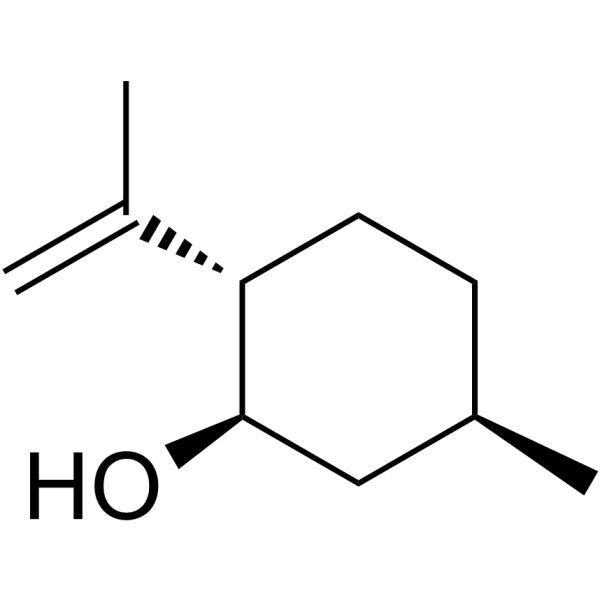 CAS#:89-79-2
CAS#:89-79-2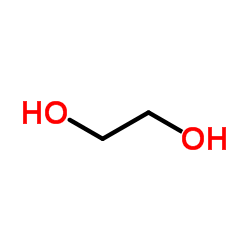 CAS#:107-21-1
CAS#:107-21-1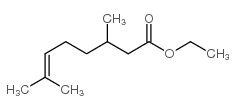 CAS#:26728-44-9
CAS#:26728-44-9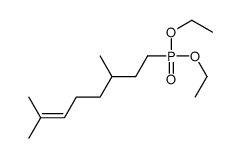 CAS#:25901-59-1
CAS#:25901-59-1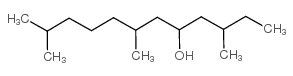 CAS#:30221-43-3
CAS#:30221-43-3
