Hyperforin
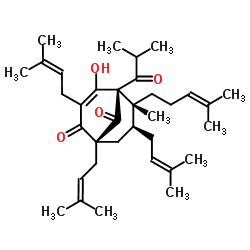
Hyperforin structure
|
Common Name | Hyperforin | ||
|---|---|---|---|---|
| CAS Number | 11079-53-1 | Molecular Weight | 536.785 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 616.8±55.0 °C at 760 mmHg | |
| Molecular Formula | C35H52O4 | Melting Point | 79-80ºC | |
| MSDS | USA | Flash Point | 340.9±28.0 °C | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of HyperforinHyperforin is a transient receptor canonical 6 (TRPC6) channels activator. Hyperforin modulates Ca2+ levels by activating Ca2+-conducting non-selective canonical TRPC6 channels. Hyperforin also shows diverse pharmacological activities including anti-depression, anti-tumor, anti-dementia, anti-diabetes. Hyperforin modulates γδ T cells to secret IL-17α, improves Imiquimod (HY-B0180)-induced psoriasis-like mice model[1][2][3][4][5]. |
| Name | hyperforin |
|---|---|
| Synonym | More Synonyms |
| Description | Hyperforin is a transient receptor canonical 6 (TRPC6) channels activator. Hyperforin modulates Ca2+ levels by activating Ca2+-conducting non-selective canonical TRPC6 channels. Hyperforin also shows diverse pharmacological activities including anti-depression, anti-tumor, anti-dementia, anti-diabetes. Hyperforin modulates γδ T cells to secret IL-17α, improves Imiquimod (HY-B0180)-induced psoriasis-like mice model[1][2][3][4][5]. |
|---|---|
| Related Catalog | |
| Target |
TRPC6[1] |
| In Vitro | Hyperforin 具有多向作用机制。它阻断配体门控 (GABA、NMDA 和 AMPA 受体) 和电压门控通道(Ca2+, K+, Na+) 的电导[2]。 Hyperforin (0.1, 1,10 μM; 2 h) 降低体外培养的小鼠脾 γδ T 细胞中 IL-17A 的表达和分泌[3]。 Hyperforin (0.1, 1,10 μM; 2 h) 抑制 TNF-α 刺激的 HaCaT 细胞中 MAPK 和 STAT3 通路的磷酸化[3]。 Hyperforin (IC50=3.7 μmol/L) 抑制 HDMEC 微血管管的形成和增殖,呈剂量依赖性,无毒性作用[4]。 Western Blot Analysis[3] Cell Line: HaCaT cells Concentration: 0.1, 1, 10 μM; with or without 10, 20 ng/mL TNF-α Incubation Time: 2 hours Result: Reduced the expressions of p-p38, p-ERK, p-JNK, and p-STAT3, especially at the dosage of 10 μM. |
| In Vivo | Hyperforin (5 mg/kg; 腹腔注射; 每天 1 次, 共 7 天) 能够改善 Imiquimod (HY-B0180) 诱导的小鼠银屑病皮损情况,抑制炎症细胞浸润和炎症因子释放[3]。 Animal Model: IMQ-induced psoriasis-like mice model[3] Dosage: 5 mg/kg Administration: Intraperitoneal injection; once daily for 7 days Result: Significantly ameliorated skin lesion throughout the treatment period, demonstrated by the reduced severity score of skin inflammation. Suppressed infiltration of CD3+ T cells and downregulated expression of Il1, Il6, Il23, Il17a, Il22, antimicrobial peptides (AMPs) in the skin lesion. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 616.8±55.0 °C at 760 mmHg |
| Melting Point | 79-80ºC |
| Molecular Formula | C35H52O4 |
| Molecular Weight | 536.785 |
| Flash Point | 340.9±28.0 °C |
| Exact Mass | 536.386536 |
| PSA | 71.44000 |
| LogP | 12.30 |
| Vapour Pressure | 0.0±4.0 mmHg at 25°C |
| Index of Refraction | 1.518 |
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P280-P302 + P352 + P312-P304 + P340 + P312-P370 + P378-P403 + P235 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | F,T |
| Risk Phrases | 11-23/24/25-39/23/24/25 |
| Safety Phrases | 7-16-36/37-45 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol |
| WGK Germany | 3 |
| HS Code | 29144000 |
|
Plant flavonol isorhamnetin attenuates chemically induced inflammatory bowel disease via a PXR-dependent pathway.
J. Nutr. Biochem. 25(9) , 923-33, (2014) Isorhamnetin is an O-methylated flavonol present in fruit and vegetables. We recently reported the identification of isorhamnetin as an activator of the human pregnane X receptor (PXR), a known target... |
|
|
Phytochemical analysis and in vitro biological activity of three Hypericum species from the Canary Islands (Hypericum reflexum, Hypericum canariense and Hypericum grandifolium).
Fitoterapia 100 , 95-109, (2015) In the present work we carried out a phytochemical and biological investigation on three Hypericum species, i.e. Hypericum reflexum, Hypericum canariense and Hypericum grandifolium, from the Canary Is... |
|
|
Human UGT1A4 and UGT1A3 conjugate 25-hydroxyvitamin D3: metabolite structure, kinetics, inducibility, and interindividual variability.
Endocrinology 155(6) , 2052-63, (2014) 25-Hydroxyvitamin D3 (25OHD3) is used as a clinical biomarker for assessment of vitamin D status. Blood levels of 25OHD3 represent a balance between its formation rate and clearance by several oxidati... |
| (1R,5S,6R,7S)-4-Hydroxy-6-methyl-1,3,7-tris(3-methyl-2-butenyl)-5-(2-methyl-1-oxopropyl)-6-(4-methyl-3-pentenyl)bicyclo[3.3.1]non-3-ene-2,9-dione |
| (1R,5S,6R,7S)-4-hydroxy-6-methyl-1,3,7-tris(3-methylbut-2-en-1-yl)-6-(4-methylpent-3-en-1-yl)-5-(2-methylpropanoyl)bicyclo[3.3.1]non-3-ene-2,9-dione |
| Hyperforin |
| (1R,5S,6R,7S)-4-Hydroxy-5-isobutyryl-6-methyl-1,3,7-tris(3-methylbut-2-en-1-yl)-6-(4-methylpent-3-en-1-yl)bicyclo[3.3.1]non-3-ene-2,9-dione |
| (1R,5S,6R,7S)-4-Hydroxy-6-methyl-1,3,7-tris(3-methylbut-2-enyl)-6-(4-methylpent-3-enyl)-5-(2-methylpropanoyl)bicyclo[3.3.1]non-3-ene-2,9-dione |
| Bicyclo[3.3.1]non-3-ene-2,9-dione, 4-hydroxy-6-methyl-1,3,7-tris(3-methyl-2-buten-1-yl)-5-(2-methyl-1-oxopropyl)-6-(4-methyl-3-penten-1-yl)-, (1R,5S,6R,7S)- |
| MFCD01861497 |
| (1R,5S,6R,7S)-4-Hydroxy-5-isobutyryl-6-methyl-1,3,7-tris(3-methyl-2-buten-1-yl)-6-(4-methyl-3-penten-1-yl)bicyclo[3.3.1]non-3-ene-2,9-dione |