Sch 39166
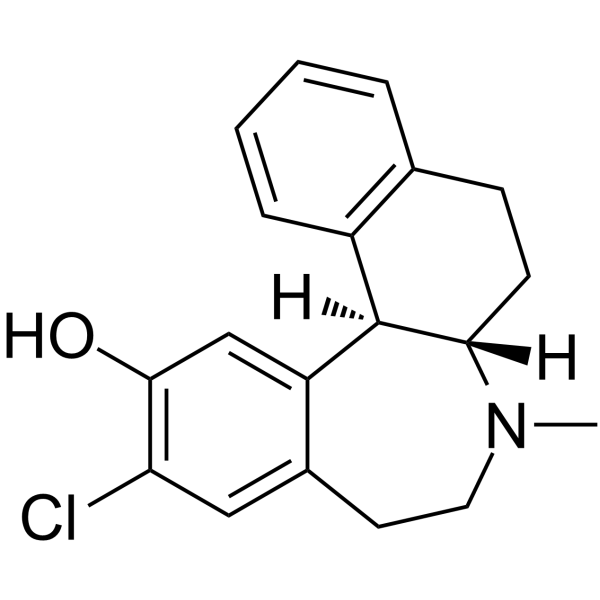
Sch 39166 structure
|
Common Name | Sch 39166 | ||
|---|---|---|---|---|
| CAS Number | 112108-01-7 | Molecular Weight | 313.82100 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C19H20ClNO | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Sch 39166Ecopipam (SCH 39166) is a potent, selective and orally active antagonist of dopamine D1/D5 receptor, with Kis of 1.2 nM and 2.0 nM, respectively. Ecopipam shows more than 40-flod selectivity over D2, D4, 5-HT, and α2a receptor (Ki=0.98, 5.52, 0.08, and 0.73 μM, respectively). Ecopipam can be used for the research of schizophrenia, cocaine addition, and obesity[1][3]. |
| Name | ecopipam |
|---|---|
| Synonym | More Synonyms |
| Description | Ecopipam (SCH 39166) is a potent, selective and orally active antagonist of dopamine D1/D5 receptor, with Kis of 1.2 nM and 2.0 nM, respectively. Ecopipam shows more than 40-flod selectivity over D2, D4, 5-HT, and α2a receptor (Ki=0.98, 5.52, 0.08, and 0.73 μM, respectively). Ecopipam can be used for the research of schizophrenia, cocaine addition, and obesity[1][3]. |
|---|---|
| Related Catalog | |
| Target |
D1 Receptor:1.2 nM (Ki) D5 Receptor:2.0 nM (Ki) D2 Receptor:980 nM (Ki) D4 Receptor:5520 nM (Ki) 5-HT Receptor:80 nM (Ki) Alpha-2A adrenergic receptor:731 nM (Ki) |
| In Vitro | Ecopipam (2 μM) completely abolishes the proconvulsive effect of Dopamine (10 μM) in isolated corticohippocampal formation[2]. |
| In Vivo | Ecopipam (0.003-0.3 mg/kg; a single s.c.) abolishes Nicotine-induced enhancement of a sensory reinforcer in adult rats[3]. Ecopipam (10, mg/kg, oral administration) antagonizes Apomorphine-induced stereotypy in rats[4]. Ecopipam (5 and 10 μM, perfusion, 1 μL/min) reversibly and dose-dependently decreases acetylcholine release in the rat striatum[5]. Animal Model: Male young adult Long-Evans rats injected with Nicotine[3] Dosage: 0.003, 0.01, 0.03, 0.1, 0.3 mg/kg Administration: A single s.c. 20 min before Nicotine (0.1 mg/kg) Result: Dose-dependently reduced pressing on both active and inactive levers. |
| References |
| Molecular Formula | C19H20ClNO |
|---|---|
| Molecular Weight | 313.82100 |
| Exact Mass | 313.12300 |
| PSA | 23.47000 |
| LogP | 3.91810 |
| SCH 39166 |
| (-)-trans-6,7,7a,8,9,13b-hexahydro-3-chloro-2-hydroxy-N-methyl-5H-benzo-[d]naphtho{2,1-b}azepine |
| (6aS,13bR)-11-chloro-6,6a,7,8,9,13b-hexahydro-7-methyl-5H-benzo[d]naphth[2,1-b]azepin-12-ol |
| (-)-Ecopipam |
| (6aS,13bR)-11-Chloro-7-methyl-5,6a,7,8,9,13b-hexahydro-6H-7-aza-benzo[6,7]cyclohepta[1,2-a]naphthalen-12-ol |
| trans-(-)-(6aS,13bR)-11-chloro-6,6a,7,8,9,13b-hexahydro-7-methyl-5H-benzo[d]naphth[2,1-b]azepin-12-ol |
| (-)-trans-6,7,7a,8,9,13b-hexahydro-3-chloro-2-hydroxy-N-methyl-5H-benzo[d]naphtho[2,1-b]azepine |