Phenamil methanesulfonate
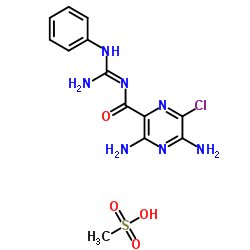
Phenamil methanesulfonate structure
|
Common Name | Phenamil methanesulfonate | ||
|---|---|---|---|---|
| CAS Number | 1161-94-0 | Molecular Weight | 401.829 | |
| Density | N/A | Boiling Point | 621.7ºC at 760 mmHg | |
| Molecular Formula | C13H16ClN7O4S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 329.8ºC | |
Use of Phenamil methanesulfonatePhenamil methanesulfonate, an analog of Amiloride (HY-B0285), is a more potent and less reversible epithelial sodium channel (ENaC) blocker with an IC50 of 400 nM[2]. Phenamil methanesulfonate is also a competive inhibitor of TRPP3 and inhibits TRPP3-mediated Ca2+ transport with an IC50 of 140 nM in a Ca2+ uptake assay[1]. Phenamil methanesulfonate is an intriguing small molecule to promote bone repair by strongly activating BMP signaling pathway[4]. Phenamil methanesulfonate is used for the research of cystic fibrosis lung disease[5]. |
| Name | phenamil methanesulfonate |
|---|---|
| Synonym | More Synonyms |
| Description | Phenamil methanesulfonate, an analog of Amiloride (HY-B0285), is a more potent and less reversible epithelial sodium channel (ENaC) blocker with an IC50 of 400 nM[2]. Phenamil methanesulfonate is also a competive inhibitor of TRPP3 and inhibits TRPP3-mediated Ca2+ transport with an IC50 of 140 nM in a Ca2+ uptake assay[1]. Phenamil methanesulfonate is an intriguing small molecule to promote bone repair by strongly activating BMP signaling pathway[4]. Phenamil methanesulfonate is used for the research of cystic fibrosis lung disease[5]. |
|---|---|
| Related Catalog | |
| Target |
TRPC3:140 nM (IC50) |
| In Vitro | TRPP3, a member of the transient receptor potential (TRP) superfamily of cation channels, is a Ca2+-activated channel permeable to Ca2+, Na+, and K+. TRPP3 is implicated in regulation of pH-sensitive action potential in spinal cord neurons. Phenamil methanesulfonate (1 μM) decreases 45Ca2+ uptake in a radiotracer uptake assay. It inhibits TRPP3-mediated Ca2+ transport with an IC50 value of 0.28 μM in oocytes expressing TRPP3 or H2O-injected oocytes[1]. Phenamil methanesulfonate is a more potent ENaC blocker than Amiloride, it inhibits the epithelial sodium channel (ENaC) with an IC50 of 400 nM (Amiloride=776 nM)[2]. Phenamil methanesulfonate inhibits basal short-circuit currents with IC50 values of 75 and 116 nM, respectively in both human and ovine bronchial epithelia cells[3]. Phenamil methanesulfonate (0-20 μM; 14 days) elevates adipogenic gene expression, PPARγ, Fabp4, and lipoprotein lipase expression in a concentration-dependent manner ,and regulates adipogenesis in C3H10T1/2 cells[4]. Phenamil methanesulfonate (0-20 μM; 7 or 14 days) modulates MC3T3-E1 osteoblastic differentiation, it increases Alkaline phosphatase (ALP) activity in MC3T3-E1 cells in a concentration-dependent manner[4]. RT-PCR[4] Cell Line: C3H10T1/2 cells Concentration: 0 μM and 20 μM Incubation Time: 14 days Result: Increased PPARγ, Fabp4, and lipoprotein lipase (LPL) mRNA expression. |
| In Vivo | Phenamil methanesulfonate (subcutaneous injection; 15 or 30 mg/kg; 21 days; infusion rate of 1 ml/h) reduces chronic-hypoxia-induced pulmonary artery hypertension (PAH). Additionally, the mRNA level of SMA, SM22, Id3, and Trb3 from the lung sample are also decreased by Phenamil under hypoxia or normoxia in rats. However, phenamil has little effect on pulmonary vasculature under physiological conditions[5]. Animal Model: Male Sprague-Dawley rats[5] Dosage: 15 or 30 mg/kg Administration: Subcutaneous injection; 15 or 30 mg/kg; 21 days; infusion rate of 1 ml/h Result: Reduced hypoxia-induced pulmonary hypertension and vascular remodeling. |
| References |
| Boiling Point | 621.7ºC at 760 mmHg |
|---|---|
| Molecular Formula | C13H16ClN7O4S |
| Molecular Weight | 401.829 |
| Flash Point | 329.8ºC |
| Exact Mass | 401.067291 |
| PSA | 205.55000 |
| LogP | 3.38190 |
| Vapour Pressure | 2.22E-15mmHg at 25°C |
| Storage condition | -20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2924299090 |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
The lung amiloride-sensitive Na+ channel: biophysical properties, pharmacology, ontogenesis, and molecular cloning.
Proc. Natl. Acad. Sci. U. S. A. 91 , 247, (1994) Water balance in the lung is controlled via active Na+ and Cl- transport. Electrophysiological measurements on lung epithelial cells demonstrated the presence of a Na+ channel that is inhibited by ami... |
|
|
Phenamil: an irreversible inhibitor of sodium channels in the toad urinary bladder.
J. Membr. Biol. 87 , 45, (1985) Several new amiloride analogues and two reported photoaffinity analogues were tested for irreversible inhibition of short-circuit current, Isc, in toad bladder. Bromoamiloride, a photoaffinity analogu... |
|
|
Biochemical identification of two types of phenamil binding sites associated with amiloride-sensitive Na+ channels.
Biochemistry 28 , 3744, (1989) The existence of distinct forms of the epithelium Na+ channel that differ in their sensitivity to amiloride has been repeatedly suggested by physiological data. The biochemical basis for these differe... |
| 2-Pyrazinecarboxamide, 3,5-diamino-N-[(1E)-amino(phenylamino)methylene]-6-chloro-, methanesulfonate (1:1) |
| Phenaethylphosphorodichloridat |
| MFCD00274070 |
| 2-Cl-IB-MECA |
| Phenamil methanesulfonate salt |
| 3,5-Diamino-N-[(E)-amino(anilino)methylene]-6-chloro-2-pyrazinecarboxamide methanesulfonate (1:1) |
| Phosphorodichloridic acid,2-phenylethyl ester |
| 3,5-Diamino-6-chloro-N-(N-phenylcarbamimidoyl)pyrazine-2-carboxamide methanesulfonate (1:1) |