Nitecapone
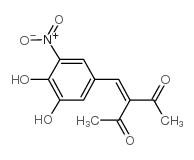
Nitecapone structure
|
Common Name | Nitecapone | ||
|---|---|---|---|---|
| CAS Number | 116313-94-1 | Molecular Weight | 265.21900 | |
| Density | 1.451g/cm3 | Boiling Point | 495.3ºC at 760 mmHg | |
| Molecular Formula | C12H11NO6 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 211.6ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of NitecaponeNitecapone (OR-462) is an orally active and short-acting catechol-O-methyltransferase (COMT) inhibitor with gastroprotective and antioxidant properties. Nitecapone (OR-462) scavenges reactive oxygen and nitric radicals and prevents lipid peroxidation[1][2][3]. |
| Name | 3-[(3,4-dihydroxy-5-nitrophenyl)methylidene]pentane-2,4-dione |
|---|---|
| Synonym | More Synonyms |
| Description | Nitecapone (OR-462) is an orally active and short-acting catechol-O-methyltransferase (COMT) inhibitor with gastroprotective and antioxidant properties. Nitecapone (OR-462) scavenges reactive oxygen and nitric radicals and prevents lipid peroxidation[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Nitecapone (1-100 μM) reducesd GSH (reduced glutathione) depletion induced by ROO-by 11-38% and oxidation to oxidized glutathione (GSSG) by 32-45%[1]. |
| In Vivo | Nitecapone (30 mg/kg, ip daily for 13 days) reduces development and symptoms of neuropathic pain after spinal nerve ligation in rats[3]. Animal Model: Eighty-six male Wistar rats, weighing 140-350 g[3]. Dosage: 30 mg/kg (3.3 mL/kg). Administration: IP, once daily for 13 days. Result: Selectively and specifically inhibits COMT in the peripheral tissues, and to some extent in the CNS for ca. 3 h. Increased the thresholds for the mechanical stimuli and thus reduced mechanical allodynia. Reduced the number of positive reactions of the ipsilateral paws when compared with the baselines in the nitecapone-pretreated rats. |
| References |
| Density | 1.451g/cm3 |
|---|---|
| Boiling Point | 495.3ºC at 760 mmHg |
| Molecular Formula | C12H11NO6 |
| Molecular Weight | 265.21900 |
| Flash Point | 211.6ºC |
| Exact Mass | 265.05900 |
| PSA | 120.42000 |
| LogP | 2.09060 |
| Vapour Pressure | 1.95E-10mmHg at 25°C |
| Index of Refraction | 1.645 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| RIDADR | UN 2811 6.1 / PGIII |
|
~45% 
Nitecapone CAS#:116313-94-1 |
| Literature: Journal of Medicinal Chemistry, , vol. 32, # 4 p. 841 - 846 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
|
D1-like dopamine receptor activation and natriuresis by nitrocatechol COMT inhibitors.
Kidney Int. 59(5) , 1683-94, (2001) In recent years, several nitrocatechol derivatives (tolcapone, entacapone, and nitecapone) have been developed and found to be highly selective and potent inhibitors of catechol-O-methyltransferase (C... |
|
|
Extending levodopa action: COMT inhibition.
Neurology 50(6 Suppl 6) , S27-32; discussion S44-8, (1998) Degradation of levodopa in the periphery is known to be associated with motor fluctuations and dyskinesia in Parkinson's disease (PD) patients. The enzyme catechol-O-methyltransferase (COMT) is respon... |
|
|
The Helicobacter felis mouse model in assessing anti-Helicobacter therapies and gastric mucosal prostaglandin E2 levels.
Scand. J. Gastroenterol. 31(4) , 334-8, (1996) The aims of the present study were to assess the usefulness of the Helicobacter felis mouse model in the evaluation of antimicrobial therapies and the effect of Helicobacter infection on gastric mucos... |
| 3-(3,4-dihydroxy-5-nitrobenzylidene)-2,4-pentanedione |
| 2,4-Pentanedione,3-((3,4-dihydroxy-5-nitrophenyl)methylene) |
| Nitecapone |
| 3-(3,4-dihydroxy-5-nitrophenyl)methylene-2,4-pentanedione |
| Nitecapona [INN-Spanish] |
| Nitecapone [INN] |
| OR 462 |
| Nitecaponum [INN-Latin] |
 CAS#:118724-88-2
CAS#:118724-88-2