Luzindole
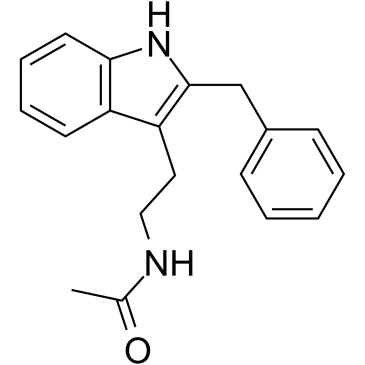
Luzindole structure
|
Common Name | Luzindole | ||
|---|---|---|---|---|
| CAS Number | 117946-91-5 | Molecular Weight | 292.375 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 559.6±38.0 °C at 760 mmHg | |
| Molecular Formula | C19H20N2O | Melting Point | 44-46°C | |
| MSDS | USA | Flash Point | 292.2±26.8 °C | |
Use of LuzindoleLuzindole (N-0774) is a selective melatonin receptor antagonist. Luzindole preferentially targets MT2 (Mel1b) over MT1 (Mel1a) with Ki values of 10.2 and 158 nM for human MT2 and MT1, respectively. Luzindole suppresses experimental autoimmune encephalomyelitis (EAE), and exerts antidepressant-like activity[1][2][3]. |
| Name | N-[2-(2-benzyl-1H-indol-3-yl)ethyl]acetamide |
|---|---|
| Synonym | More Synonyms |
| Description | Luzindole (N-0774) is a selective melatonin receptor antagonist. Luzindole preferentially targets MT2 (Mel1b) over MT1 (Mel1a) with Ki values of 10.2 and 158 nM for human MT2 and MT1, respectively. Luzindole suppresses experimental autoimmune encephalomyelitis (EAE), and exerts antidepressant-like activity[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Ki :10.2 nM (human MT2), 158 nM (human MT1)[1] |
| In Vitro | Luzindole (N-0774) (5-10 μg/ml) inhibits antigen-specific proliferation of the MBP-reactive LV-4 T cell line[1]. |
| In Vivo | Luzindole (N-0774) (30 mg/kg; i.p.; days 0-5) suppresses experimental autoimmune encephalomyelitis[2]. Luzindole (N-0774) (30 mg/kg i.p.) reduces the time of immobility in a dose-dependent manner, the effect being more pronounced at midnight (60% reduction) than at noon (39% reduction). The effect of luzindole is time-dependent, showing a maximal effect at 60 min. The anti-immobility effect of luzindole (10 mg/kg i.p.) is prevented by the administration of melatonin (30 mg/kg i.p.). Luzindole (30 mg/kg i.p.) did not modify the time of immobility either at noon or midnight in the albino ND/4 mouse, or in the C57BL/6J mouse, which does not produce melatonin[3]. Animal Model: Twenty-three- to 12-week-old(SJL X PL/J ) F1 mice[2] Dosage: 30 mg/kg Administration: i.p.; days 0-5 (between 23: 00 and 1: 00 under conditions of minimal lighting) Result: Effectively prevented experimental autoimmune encephalomyelitis. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 559.6±38.0 °C at 760 mmHg |
| Melting Point | 44-46°C |
| Molecular Formula | C19H20N2O |
| Molecular Weight | 292.375 |
| Flash Point | 292.2±26.8 °C |
| Exact Mass | 292.157562 |
| PSA | 44.89000 |
| LogP | 3.04 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.632 |
| Storage condition | −20°C |
| Stability | Store at -20°C |
| Water Solubility | DMSO: 5 mg/mL |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
~% 
Luzindole CAS#:117946-91-5 |
| Literature: US5093352 A1, ; US 5093352 A |
|
~73% 
Luzindole CAS#:117946-91-5 |
| Literature: Journal of Organic Chemistry, , vol. 77, # 14 p. 6351 - 6357 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
|
Inhibitory effect of melatonin on testosterone synthesis is mediated via GATA-4/SF-1 transcription factors.
Reprod. Biomed. Online 31 , 638-46, (2015) The aim of the present study was to elucidate whether the GATA-4/SF-1 signalling pathway is involved in the inhibitory effects of melatonin on testosterone production in both the TM3 Leydig cell line ... |
|
|
Selective protection of the cerebellum against intracerebroventricular LPS is mediated by local melatonin synthesis.
Brain Struct. Funct. 220(2) , 827-40, (2015) Although melatonin is mainly produced by the pineal gland, an increasing number of extra-pineal sites of melatonin synthesis have been described. We previously demonstrated the existence of bidirectio... |
|
|
The influence od melatonin receptors antagonists, luzindole and 4-phenyl-2-propionamidotetralin (4-P-PDOT), on melatonin-dependent vasopressin and adrenocorticotropic hormone (ACTH) release from the rat hypothalamo-hypophysial system. In vitro and in vivo studies.
J. Physiol. Pharmacol. 65(6) , 777-84, (2015) Melatonin exerts its biological role acting via G protein-coupled membrane receptors - MT1 and MT2, as well as through cytoplasmic and/or nuclear receptors. Melatonin has previously been shown to chan... |
| N-[2-(2-Benzyl-1H-indol-3-yl)ethyl]acetamide |
| Luzindole |
| Acetamide, N-[2-[2-(phenylmethyl)-1H-indol-3-yl]ethyl]- |
| N-Acetyl-2-benzyltryptamine |
| 2-Benzyl-N-acetyltryptamine |
| N-0774 |
| N-Acetyl-2-benzyltryptamine N-[2-[2-(Phenylmethyl)-1H-indol-3-yl]ethyl]acetamide |
| MFCD00672498 |
| Arachidonyl serotonin |
| Tocris-0877 |