CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
GN6550000
-
CHEMICAL NAME :
-
Coumarin, 6,7-dimethoxy-
-
CAS REGISTRY NUMBER :
-
120-08-1
-
BEILSTEIN REFERENCE NO. :
-
0169572
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
6
-
MOLECULAR FORMULA :
-
C11-H10-O4
-
MOLECULAR WEIGHT :
-
206.21
-
WISWESSER LINE NOTATION :
-
T66 BOVJ HO1 IO1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
292 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
DRFUD4 Drugs of the Future. (J.R. Prous, S.A., Apartado de Correos 540, 08080 Barcelona, Spain) V.1- 1975/76- Volume(issue)/page/year: 3,550,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
190 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
DRFUD4 Drugs of the Future. (J.R. Prous, S.A., Apartado de Correos 540, 08080 Barcelona, Spain) V.1- 1975/76- Volume(issue)/page/year: 3,550,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
280 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
DRFUD4 Drugs of the Future. (J.R. Prous, S.A., Apartado de Correos 540, 08080 Barcelona, Spain) V.1- 1975/76- Volume(issue)/page/year: 3,550,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
180 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Behavioral - alteration of classical conditioning Nutritional and Gross Metabolic - body temperature decrease
-
REFERENCE :
-
IJMRAQ Indian Journal of Medical Research. (Indian Council of Medical Research, Ansari Nagar, New Delhi 110 029, India) V.1- 1913- Volume(issue)/page/year: 60,763,1972 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1180 mg/kg
-
SEX/DURATION :
-
male 8 week(s) pre-mating female 2 week(s) pre-mating - 3 week(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated) Reproductive - Effects on Newborn - delayed effects
-
REFERENCE :
-
IJEBA6 Indian Journal of Experimental Biology. (Publications & Information Directorate, CSIR, Hillside Rd., New Delhi 110 012, India) V.1- 1963- Volume(issue)/page/year: 17,740,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
725 mg/kg
-
SEX/DURATION :
-
female 15-22 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
IJEBA6 Indian Journal of Experimental Biology. (Publications & Information Directorate, CSIR, Hillside Rd., New Delhi 110 012, India) V.1- 1963- Volume(issue)/page/year: 17,740,1979
|
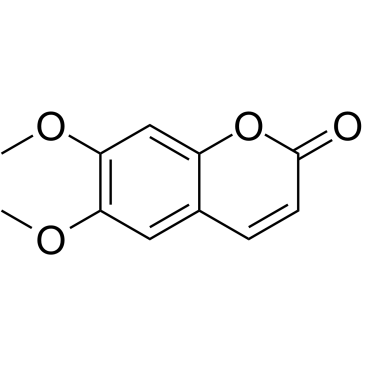


 CAS#:305-01-1
CAS#:305-01-1 CAS#:77-78-1
CAS#:77-78-1 CAS#:74-88-4
CAS#:74-88-4 CAS#:2033-89-8
CAS#:2033-89-8 CAS#:15568-85-1
CAS#:15568-85-1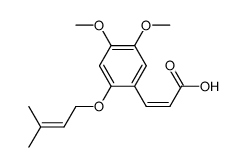 CAS#:73490-50-3
CAS#:73490-50-3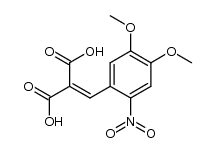 CAS#:109300-68-7
CAS#:109300-68-7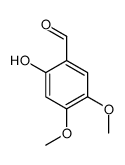 CAS#:14382-91-3
CAS#:14382-91-3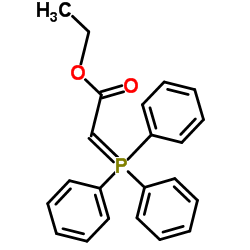 CAS#:1099-45-2
CAS#:1099-45-2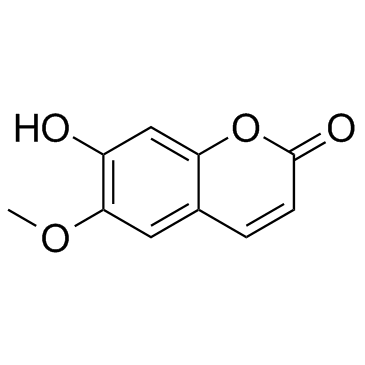 CAS#:92-61-5
CAS#:92-61-5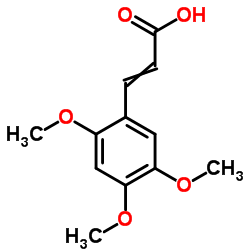 CAS#:24160-53-0
CAS#:24160-53-0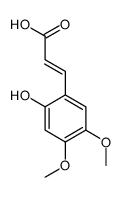 CAS#:114515-53-6
CAS#:114515-53-6 CAS#:56680-28-5
CAS#:56680-28-5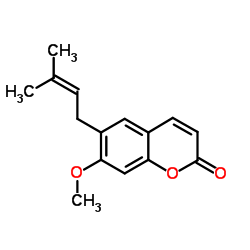 CAS#:581-31-7
CAS#:581-31-7
