PS315
Modify Date: 2024-01-10 19:18:31
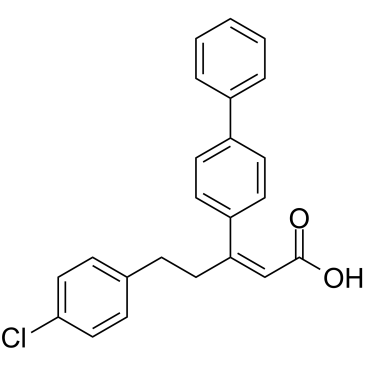
PS315 structure
|
Common Name | PS315 | ||
|---|---|---|---|---|
| CAS Number | 1221964-50-6 | Molecular Weight | 362.85 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C23H19ClO2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of PS315PS315, a derivative of PS48 (HY-15967), is an allosteric PKC inhibitor by binding to the PIF-pocket of aPKC and inducing a displacement of the active site residue Lys111. PS315 inhibits the full-length and catalytic domain constructs of PKCζ (IC50=10 μM) and PKCη (IC50=30 μM). PS315 has anti-cancer activity[1]. |
| Name | PS315 |
|---|
| Description | PS315, a derivative of PS48 (HY-15967), is an allosteric PKC inhibitor by binding to the PIF-pocket of aPKC and inducing a displacement of the active site residue Lys111. PS315 inhibits the full-length and catalytic domain constructs of PKCζ (IC50=10 μM) and PKCη (IC50=30 μM). PS315 has anti-cancer activity[1]. |
|---|---|
| Related Catalog | |
| Target |
PKCζ:10 μM (IC50) PKCη:30 μM (IC50) |
| In Vitro | Preincubation of U937 cells with 5 μM PS315 inhibits TNF-α induced NF-κB activation by 74%, whereas complete inhibition is observed with 10 μM PS315[1]. The small allosteric inhibitor PS315 and the N-terminal region of aPKC both act directly on the PIF-pocket on-off switch. PS315, binding at the PIF-pocket, induces a displacement of the active site residue Lys111, thereby inhibiting the activity of aPKCs by allosterically affecting the catalytic mechanism of the kinase[1]. |
| References |
| Molecular Formula | C23H19ClO2 |
|---|---|
| Molecular Weight | 362.85 |