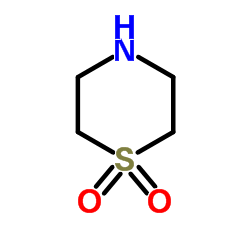
Thiomorpholine
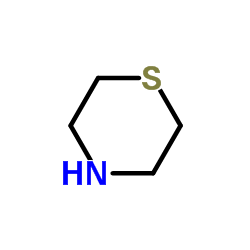
Thiomorpholine structure
|
Common Name | Thiomorpholine | ||
|---|---|---|---|---|
| CAS Number | 123-90-0 | Molecular Weight | 103.19 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 170.0±15.0 °C at 760 mmHg | |
| Molecular Formula | C4H9NS | Melting Point | 166-168 | |
| MSDS | Chinese USA | Flash Point | 60.0±0.0 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
Use of ThiomorpholineThiomorpholine is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | thiomorpholine |
|---|---|
| Synonym | More Synonyms |
| Description | Thiomorpholine is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 170.0±15.0 °C at 760 mmHg |
| Melting Point | 166-168 |
| Molecular Formula | C4H9NS |
| Molecular Weight | 103.19 |
| Flash Point | 60.0±0.0 °C |
| Exact Mass | 103.045570 |
| PSA | 37.33000 |
| LogP | 0.27 |
| Vapour Pressure | 1.5±0.3 mmHg at 25°C |
| Index of Refraction | 1.499 |
| Symbol |

GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H314 |
| Precautionary Statements | P280-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | C:Corrosive; |
| Risk Phrases | R34;R37 |
| Safety Phrases | S26-S36/37/39-S45 |
| RIDADR | UN 3267 8/PG 3 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 8 |
| HS Code | 2934999090 |
|
~% 
Thiomorpholine CAS#:123-90-0 |
| Literature: Journal of the American Chemical Society, , vol. 76, p. 1187 |
|
~% 
Thiomorpholine CAS#:123-90-0 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 57, # 12 p. 1415 - 1420 |
|
~% 
Thiomorpholine CAS#:123-90-0 |
| Literature: Journal of the Chemical Society, , vol. 117, p. 299 |
|
~% 
Thiomorpholine CAS#:123-90-0 |
| Literature: Journal of Organic Chemistry, , vol. 73, # 18 p. 7189 - 7196 |
|
~% 
Thiomorpholine CAS#:123-90-0 |
| Literature: Australian Journal of Chemistry, , vol. 9, p. 397,402 Chim. moderne, , vol. 4, p. 53 - 55 |
|
~% 
Thiomorpholine CAS#:123-90-0 |
| Literature: Bihang till Svenska Vet.-Akad. Handlingar 22 II, No. 1, S. 5 |
|
~% 
Thiomorpholine CAS#:123-90-0 |
| Literature: Australian Journal of Chemistry, , vol. 9, p. 397,402 |
|
~% 
Thiomorpholine CAS#:123-90-0 |
| Literature: Bihang till Svenska Vet.-Akad.Handlingar, vol. 22, p. II,No.1,S.5 |
| Precursor 7 | |
|---|---|
| DownStream 9 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Synthesis and antitumor activities of some new N1-(flavon-6-yl)amidrazone derivatives.
Arch. Pharm. (Weinheim) 347(6) , 415-22, (2014) A new series of N1-(flavon-6-yl)amidrazones were synthesized by reacting the hydrazonoyl chloride derived from 6-aminoflavone with the appropriate sec-cyclic amines. The antitumor activities of these ... |
|
|
Structure-activity relationships of a novel pyranopyridine series of Gram-negative bacterial efflux pump inhibitors.
Bioorg. Med. Chem. 23(9) , 2024-34, (2015) Recently we described a novel pyranopyridine inhibitor (MBX2319) of RND-type efflux pumps of the Enterobacteriaceae. MBX2319 (3,3-dimethyl-5-cyano-8-morpholino-6-(phenethylthio)-3,4-dihydro-1H-pyrano[... |
|
|
Redox-neutral α-sulfenylation of secondary amines: ring-fused N,S-acetals.
Org. Lett. 16(13) , 3556-9, (2014) Secondary amines react with thiosalicylaldehydes in the presence of catalytic amounts of acetic acid to generate ring-fused N,S-acetals in redox-neutral fashion. A broad range of amines undergo α-sulf... |
| T6M DSTJ |
| 1-Thia-4-azacyclohexane |
| perhydro-1,4-thiazine |
| EINECS 204-660-2 |
| Thiomorpholine |
| UNII-3A8R61G6QV |
| Tetrahydro-2H-1,4-thiazine,Thiamorpholine |
| thiamorpholine |
| Thiazolidinane |
| parathiazan |
| 1,4-Thiazane |
| Tetrahydro-2H-1,4-thiazine |
| [1,4]thiazinane |
| MFCD00005974 |
| 1,4-Thiazan |
| tetrahydro-1,4-thiazine |


![3-BROMO-6,7-DIHYDRO-5H-PYRROLO[3,4-B]PYRIDINE structure](https://image.chemsrc.com/caspic/189/334-22-5.png)

 CAS#:39093-93-1
CAS#:39093-93-1 CAS#:220655-09-4
CAS#:220655-09-4 CAS#:223556-42-1
CAS#:223556-42-1 CAS#:67-51-6
CAS#:67-51-6 CAS#:26541-51-5
CAS#:26541-51-5 CAS#:640-61-9
CAS#:640-61-9 CAS#:1060936-14-2
CAS#:1060936-14-2 CAS#:6007-64-3
CAS#:6007-64-3![4-[Dimethyl(vinyl)silyl]thiomorpholine structure](https://image.chemsrc.com/caspic/209/1000598-42-4.png) CAS#:1000598-42-4
CAS#:1000598-42-4
