| Description |
Etomoxir ((R)-(+)-Etomoxir) is a potent inhibitor of carnitine palmitoyltransferase-I (CPT-1).
|
| Related Catalog |
|
| Target |
CPT-1[1]
|
| In Vitro |
Etomoxir binds irreversibly to the catalytic site of CPT-1 inhibiting its activity, but also upregulates fatty acid oxidation enzymes. Etomoxir is developed as an inhibitor of the mitochondrial carnitine palmitoyltransferase-1 (CPT-1) located on the outer mitochondrial membrane. Etomoxir, in the liver can act as peroxisomal proliferator, increasing DNA synthesis and liver growth. Thus, etomoxir, in addition of being a CPT1 inhibitor could be considered as a PPARalpha agonist[1]. Etomoxir is a member of the oxirane carboxylic acid carnitine palmitoyl transferase I inhibitors and has been suggested as a therapeutic agent for the treatment of heart failure. Acute Etomoxir treatment irreversibly inhibits the activity of carnitine palmitoyltransferase I. As a result, fatty acid import into the mitochondria and β-oxidation is reduced, whereas cytosolic fatty acid accumulates and glucose oxidation is elevated. Prolonged incubation (24 h) with Etomoxir produces diverse effects on the expression of several metabolic enzyme[2].
|
| In Vivo |
Etomoxir is an inhibitor of free fatty acid (FFA) oxidation-related key enzyme CPT1. P53 interacts directly with Bax, which is inhibited by Etomoxir, further confirming the direct interaction of P53 and Bax, and the involvement of FAO-mediated mitochondrial ROS generation in db/db mice[3]. Rats are injected daily with Etomoxir, a specific CPT-I inhibitor, for 8 days at 20 mg/kg of body mass. Etomoxir-treated rats display a 44% reduced cardiac CPT-I activity. The treatment of Lewis rats for 8 days with 20 mg/kg Etomoxir does not alter blood glucose, which is in line with comparable etomoxir-feeding studies. Similarly, Etomoxir feeding does not affect general growth characteristics such as gain in body mass, nor does it affect hindlimb muscle mass. However, heart mass and liver mass are both significantly increased by 11% in Etomoxir-treated rats[4].
|
| Cell Assay |
Rat heart H9c2 myoblastic cells are incubated in DMEM containing 10% fetal bovine serum until near confluence. In some experiments, cells are preincubated for 2 h with DMEM (serum-free) in the absence or presence of 1-80 μM Etomoxir and then incubated for 2 h with 0.1 mM [1-14C]oleic acid (10 μCi/dish, binds to BSA in a 1:1 molar ratio). In other experiments, cells are preincubated for 2 h plus or minus 40 μM Etomoxir and then incubated for 2 h with 0.1 μM or 0.1 mM [1,3-3H]glycerol (10 μCi/dish), 0.1 mM [1-14C]oleic acid (2 μCi/dish, binds to BSA in a 1:1 molar ratio), 0.1 mM [1-14C]palmitic acid (2 μCi/dish, binds to BSA in a 1:1 molar ratio), 28 μM [3H]ethanolamine (2 μCi/dish), 28 μM [methyl-3H]choline (2 μCi/dish), 0.4 mM [3H]serine (20 μCi/dish), or 40 μM myo-[3H]inositol (10 μCi/dish). The medium is removed and the cells washed twice with ice-cold saline and then harvested from the dish with 2 mL methanol-water (1:1, v/v) for lipid extraction. An aliquot of the homogenate is taken for the determination of total uptake of radioactivity into cells. Phospholipids are then isolated and radioactivity in these determined[2].
|
| Animal Admin |
Mice[3] 80 male C57BLKS/J lar-Leprdb/db mice and 20 wild type littermates (8 week) are used. db/db mice are randomly divided into four groups: db/db group, Etomoxir group, MitoQ group, and PFT-α group. In the Etomoxir group, mice are intraperitoneally injected with 1 mg/kg Etomoxir twice every week. In the MitoQ group, 50 μM MitoQ is given to the mice in water. Water bottles, containing either MitoQ, are covered with aluminum foil, and all bottles are refilled every 3 days. In the PFT-α group, mice are intraperitoneally injected with 1 mg/kg PFT-α twice every week. WT mice are administrated with vehicle instead. The experimental period is 8 weeks. At the end, peripheral blood samples and bone marrow cells are harvested for the assays. Rats[4] Male Lewis rats, weighing 150-200 g, are used in the present study. Animals are kept on a 12 h:12 h light/dark cycle and fed a Purina Chow diet and water ad libitum. The rats are divided into two groups: (1) control and (2) Etomoxir. Etomoxir (20 mg/kg of body weight) is dissolved in 0.9% (w/v) NaCl and administered intraperitoneally for 8 days. Control rats receive saline. The last injection is given 24 h before the experiment. Animals are anaesthetized with an intraperitoneal injection of a nembutal and heparin (3:1) mixture. Subsequently, the heart is removed for LCFA uptake studies and for analyses of transporter protein contents.
|
| References |
[1]. Rupp H, et al. The use of partial fatty acid oxidation inhibitors for metabolic therapy of angina pectoris and heart failure. Herz. 2002 Nov;27(7):621-36. [2]. Xu FY, et al. Etomoxir mediates differential metabolic channeling of fatty acid and glycerol precursors into cardiolipin in H9c2 cells. J Lipid Res. 2003 Feb;44(2):415-23. [3]. Li J, et al. FFA-ROS-P53-mediated mitochondrial apoptosis contributes to reduction of osteoblastogenesis and bone mass in type 2 diabetes mellitus. Sci Rep. 2015 Jul 31;5:12724. [4]. Luiken JJ, et al. Etomoxir-induced partial carnitine palmitoyltransferase-I (CPT-I) inhibition in vivo does not alter cardiac long-chain fatty acid uptake and oxidation rates. Biochem J. 2009 Apr 15;419(2):447-55.
|
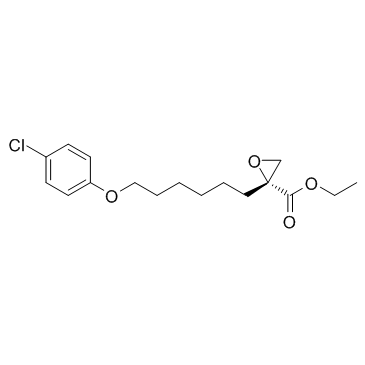
 CAS#:106-48-9
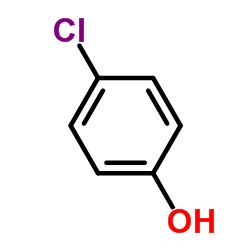
CAS#:106-48-9 CAS#:191412-56-3
CAS#:191412-56-3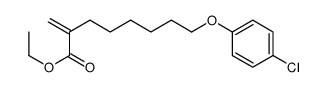 CAS#:82258-37-5
CAS#:82258-37-5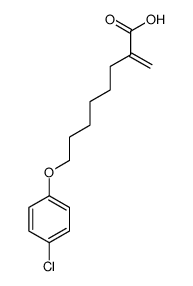 CAS#:124083-17-6
CAS#:124083-17-6 CAS#:37136-99-5
CAS#:37136-99-5![ethyl (R)-2,3-dihydroxy-2-[6-(p-chlorophenoxy)hexyl]propanoate Structure](https://image.chemsrc.com/caspic/079/314263-48-4.png) CAS#:314263-48-4
CAS#:314263-48-4 CAS#:4286-55-9
CAS#:4286-55-9
