Tolterodine-L-tartrate
Modify Date: 2024-01-02 14:02:29
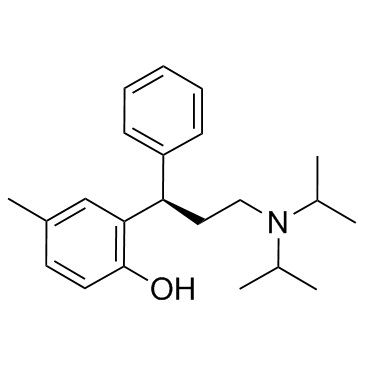
Tolterodine-L-tartrate structure
|
Common Name | Tolterodine-L-tartrate | ||
|---|---|---|---|---|
| CAS Number | 124937-51-5 | Molecular Weight | 325.488 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 442.2±45.0 °C at 760 mmHg | |
| Molecular Formula | C22H31NO | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 192.1±27.4 °C | |
Use of Tolterodine-L-tartrateTolterodine(PNU-200583) is a potent muscarinic receptor antagonists that show selectivity for the urinary bladder over salivary glands in vivo. IC50 Value:Target: mAChRin vitro: Carbachol-induced contractions of isolated guinea pig bladder were effectively inhibited by tolterodine (IC50 14 nM) and 5-HM (IC50 5.7 nM). The IC50 values were in the microM range and the antimuscarinic potency of tolterodine was 27, 200 and 370-485 times higher, respectively, than its potency in blocking histamine receptors, alpha-adrenoceptors and calcium channels. The active metabolite, 5-HM, was >900 times less potent at these sites than at bladder muscarinic receptors [1].in vivo: Tolterodine was extensively metabolized in vivo [2]. In the passive-avoidance test, tolterodine at 1 or 3 mg/kg had no effect on memory; the latency to cross and percentage of animals crossing were comparable to controls. In contrast, scopolamine induced a memory deficit; the latency to cross was decreased, and the number of animals crossing was increased [3]. |
| Name | tolterodine |
|---|---|
| Synonym | More Synonyms |
| Description | Tolterodine(PNU-200583) is a potent muscarinic receptor antagonists that show selectivity for the urinary bladder over salivary glands in vivo. IC50 Value:Target: mAChRin vitro: Carbachol-induced contractions of isolated guinea pig bladder were effectively inhibited by tolterodine (IC50 14 nM) and 5-HM (IC50 5.7 nM). The IC50 values were in the microM range and the antimuscarinic potency of tolterodine was 27, 200 and 370-485 times higher, respectively, than its potency in blocking histamine receptors, alpha-adrenoceptors and calcium channels. The active metabolite, 5-HM, was >900 times less potent at these sites than at bladder muscarinic receptors [1].in vivo: Tolterodine was extensively metabolized in vivo [2]. In the passive-avoidance test, tolterodine at 1 or 3 mg/kg had no effect on memory; the latency to cross and percentage of animals crossing were comparable to controls. In contrast, scopolamine induced a memory deficit; the latency to cross was decreased, and the number of animals crossing was increased [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 442.2±45.0 °C at 760 mmHg |
| Molecular Formula | C22H31NO |
| Molecular Weight | 325.488 |
| Flash Point | 192.1±27.4 °C |
| Exact Mass | 325.240570 |
| PSA | 23.47000 |
| LogP | 5.77 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.548 |
| Storage condition | -20°C Freezer |
| Hazard Codes | Xn,Xi |
|---|---|
| Risk Phrases | R22:Harmful if swallowed. |
| Safety Phrases | S36/37/39 |
| RIDADR | 1987 |
| WGK Germany | 2 |
| Precursor 7 | |
|---|---|
| DownStream 2 | |
| MFCD07771985 |
| (+)-Tolterodine |
| 2-[(1R)-3-(Diisopropylamino)-1-phenylpropyl]-4-methylphenol |
| (R)-2-[3-[Bis(1-methylethyl)amino]-1-phenylpropyl]-4-methylphenol |
| (R)-Tolterodine |
| tolterodine |
| Detrol |
| Tolterodine L-tartrate |
| Detrusitol |
| Phenol, 2-((1R)-3-(bis(1-methylethyl)amino)-1-phenylpropyl)-4-methyl- |
| TOLTERODINE TARTRATE |
| (+)-N,N-diisopropyl-3-(2-hydroxy-5-methylphenyl)-3-phenylpropylamine |
| (+)-(R)-2-[a-[2-(Diisopropylamino)ethyl]benzyl]-p-cresol |
| Phenol, 2-[(1R)-3-[bis(1-methylethyl)amino]-1-phenylpropyl]-4-methyl- |
 CAS#:828933-86-4
CAS#:828933-86-4 CAS#:108-18-9
CAS#:108-18-9 CAS#:848768-06-9
CAS#:848768-06-9 CAS#:40546-94-9
CAS#:40546-94-9![2-[3-[Bis(1-methylethyl)amino]-1-phenyl-propyl]-4-methyl-methoxybenzene fumarate Structure](https://image.chemsrc.com/caspic/170/124935-88-2.png) CAS#:124935-88-2
CAS#:124935-88-2 CAS#:106-44-5
CAS#:106-44-5 CAS#:827007-19-2
CAS#:827007-19-2 CAS#:207679-81-0
CAS#:207679-81-0 CAS#:286930-02-7
CAS#:286930-02-7