Evolocumab
Modify Date: 2024-01-06 10:58:37
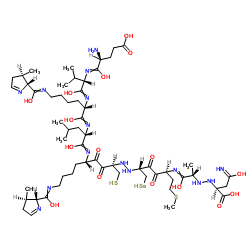
Evolocumab structure
|
Common Name | Evolocumab | ||
|---|---|---|---|---|
| CAS Number | 1256937-27-5 | Molecular Weight | 1403.572 | |
| Density | N/A | Boiling Point | 1364.0±75.0 °C at 760 mmHg | |
| Molecular Formula | C58H97N15O16S2Se | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 778.7±37.1 °C | |
Use of EvolocumabEvolocumab (AMG 145) is a human monoclonal antibody that inhibits PCSK9. Evolocumab binds to the circulating PCSK9 protein, inhibiting it from binding to the LDLR. Evolocumab can be used for the research of hypercholesterolemia and atherosclerotic cardiovascular diseases[1]. |
| Name | (2S,5S,8S,11R,14R,17S,20S,23S,26S,29S)-29-Amino-6,19,22,25,28-pentahydroxy-2-(2-hydroxy-2-iminoethyl)-17,23-bis[4-({hydroxy[(2R,3R)-3-methyl-3,4-dihydro-2H-pyrrol-2-yl]methylene}amino)butyl]-20-isobutyl-26-isopropyl-5-methyl-8-[2-(methylsulfanyl)ethyl]-9,10,15,16-tetraoxo-11-(selanylmethyl)-14-(sulfanylmethyl)-3,4,7,12,13,18,21,24,27-nonaazadotriaconta-6,18,21,24,27-pentaene-1,32-dioic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Evolocumab (AMG 145) is a human monoclonal antibody that inhibits PCSK9. Evolocumab binds to the circulating PCSK9 protein, inhibiting it from binding to the LDLR. Evolocumab can be used for the research of hypercholesterolemia and atherosclerotic cardiovascular diseases[1]. |
|---|---|
| Related Catalog | |
| Target |
PCSK9[1] |
| References |
| Boiling Point | 1364.0±75.0 °C at 760 mmHg |
|---|---|
| Molecular Formula | C58H97N15O16S2Se |
| Molecular Weight | 1403.572 |
| Flash Point | 778.7±37.1 °C |
| Exact Mass | 1403.584473 |
| LogP | 3.66 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| 3,4,7,12,13,18,21,24,27-Nonaazadotriaconta-6,18,21,24,27-pentaene-1,32-dioic acid, 29-amino-17,23-bis[4-[[[(2R,3R)-3,4-dihydro-3-methyl-2H-pyrrol-2-yl]hydroxymethylene]amino]butyl]-6,19,22,25,28-pentahydroxy-2-(2-hydroxy-2-iminoethyl)-14-(mercaptomethyl)-5-methyl-26-(1-methylethyl)-20-(2-methylpropyl)-8-[2-(methylthio)ethyl]-9,10,15,16-tetraoxo-11-(selenylmethyl)-, (2S,5S,8S,11R,14R,17S,20S,23S,26S,29S)- |
| (2S,5S,8S,11R,14R,17S,20S,23S,26S,29S)-29-Amino-6,19,22,25,28-pentahydroxy-2-(2-hydroxy-2-iminoethyl)-17,23-bis[4-({hydroxy[(2R,3R)-3-methyl-3,4-dihydro-2H-pyrrol-2-yl]methylene}amino)butyl]-20-isobutyl-26-isopropyl-5-methyl-8-[2-(methylsulfanyl)ethyl]-9,10,15,16-tetraoxo-11-(selanylmethyl)-14-(sulfanylmethyl)-3,4,7,12,13,18,21,24,27-nonaazadotriaconta-6,18,21,24,27-pentaene-1,32-dioic acid |