CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
WF1300000
-
CHEMICAL NAME :
-
Solasod-5-en-3-beta-ol
-
CAS REGISTRY NUMBER :
-
126-17-0
-
BEILSTEIN REFERENCE NO. :
-
0094578
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
14
-
MOLECULAR FORMULA :
-
C27-H43-N-O2
-
MOLECULAR WEIGHT :
-
413.71
-
WISWESSER LINE NOTATION :
-
T F5 E5 B666 HXO OUTJ A1 E1 G1 RQ H-& BT6MXTJ E1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4978 mg/kg
-
TOXIC EFFECTS :
-
Autonomic Nervous System - other (direct) parasympathomimetic Behavioral - changes in motor activity (specific assay) Kidney, Ureter, Bladder - urine volume increased
-
REFERENCE :
-
PCJOAU Pharmaceutical Chemistry Journal (English Translation). Translation of KHFZAN. (Plenum Pub. Corp., 233 Spring St., New York, NY 10013) No.1- 1967- Volume(issue)/page/year: 11,1095,1977
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
396 mg/kg
-
TOXIC EFFECTS :
-
Autonomic Nervous System - other (direct) parasympathomimetic Behavioral - changes in motor activity (specific assay) Kidney, Ureter, Bladder - urine volume increased
-
REFERENCE :
-
PCJOAU Pharmaceutical Chemistry Journal (English Translation). Translation of KHFZAN. (Plenum Pub. Corp., 233 Spring St., New York, NY 10013) No.1- 1967- Volume(issue)/page/year: 11,1095,1977
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
27500 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
FATOAO Farmakologiya i Toksikologiya (Moscow). For English translation, see PHTXA6 and RPTOAN. (V/O Mezhdunarodnaya Kniga, 113095 Moscow, USSR) V.2- 1939- Volume(issue)/page/year: 24,469,1961
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
899 mg/kg
-
TOXIC EFFECTS :
-
Autonomic Nervous System - other (direct) parasympathomimetic Behavioral - changes in motor activity (specific assay) Kidney, Ureter, Bladder - urine volume increased
-
REFERENCE :
-
PCJOAU Pharmaceutical Chemistry Journal (English Translation). Translation of KHFZAN. (Plenum Pub. Corp., 233 Spring St., New York, NY 10013) No.1- 1967- Volume(issue)/page/year: 11,1095,1977
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
103 mg/kg
-
TOXIC EFFECTS :
-
Autonomic Nervous System - other (direct) parasympathomimetic Behavioral - changes in motor activity (specific assay) Kidney, Ureter, Bladder - urine volume increased
-
REFERENCE :
-
FATOAO Farmakologiya i Toksikologiya (Moscow). For English translation, see PHTXA6 and RPTOAN. (V/O Mezhdunarodnaya Kniga, 113095 Moscow, USSR) V.2- 1939- Volume(issue)/page/year: 37,719,1974
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - hamster
-
DOSE/DURATION :
-
1200 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 17,327,1978 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4250 mg/kg/4W-I
-
TOXIC EFFECTS :
-
Brain and Coverings - other degenerative changes Kidney, Ureter, Bladder - interstitial nephritis Related to Chronic Data - death
-
REFERENCE :
-
FATOAO Farmakologiya i Toksikologiya (Moscow). For English translation, see PHTXA6 and RPTOAN. (V/O Mezhdunarodnaya Kniga, 113095 Moscow, USSR) V.2- 1939- Volume(issue)/page/year: 37,719,1974
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3375 mg/kg/5W-I
-
TOXIC EFFECTS :
-
Liver - hepatitis (hepatocellular necrosis), zonal Liver - changes in liver weight Blood - changes in leukocyte (WBC) count
-
REFERENCE :
-
PCJOAU Pharmaceutical Chemistry Journal (English Translation). Translation of KHFZAN. (Plenum Pub. Corp., 233 Spring St., New York, NY 10013) No.1- 1967- Volume(issue)/page/year: 11,1095,1977
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
120 mg/kg/8D-I
-
TOXIC EFFECTS :
-
Related to Chronic Data - death
-
REFERENCE :
-
FATOAO Farmakologiya i Toksikologiya (Moscow). For English translation, see PHTXA6 and RPTOAN. (V/O Mezhdunarodnaya Kniga, 113095 Moscow, USSR) V.2- 1939- Volume(issue)/page/year: 24,469,1961 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
120 mg/kg
-
SEX/DURATION :
-
female 6-9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
LANCAO Lancet. (7 Adam St., London WC2N 6AD, UK) V.1- 1823- Volume(issue)/page/year: 1,1187,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
300 mg/kg
-
SEX/DURATION :
-
male 30 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count) Reproductive - Paternal Effects - testes, epididymis, sperm duct
-
REFERENCE :
-
IJANDP International Journal of Andrology. (Scriptor Publisher ApS, 15 Gasvaerksvej, DK-1656 Copenhagen V, Denmark) V.1- 1978- Volume(issue)/page/year: 5,295,1982
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
15 gm/kg
-
SEX/DURATION :
-
male 150 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count) Reproductive - Paternal Effects - testes, epididymis, sperm duct Reproductive - Paternal Effects - prostate, seminal vesicle, Cowper's gland, accessory glands
-
REFERENCE :
-
ANDRDQ Andrologia. (Grosse Verlag GmbH, Kurfuerstenstr. 112-113, D-1000 Berlin 30, Fed. Rep. Ger.) V.6- 1974- Volume(issue)/page/year: 21,542,1989
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1500 mg/kg
-
SEX/DURATION :
-
female 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
BECTA6 Bulletin of Environmental Contamination and Toxicology. (Springer-Verlag New York, Inc., Service Center, 44 Hartz Way, Secaucus, NJ 07094) V.1- 1966- Volume(issue)/page/year: 15,522,1976
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1184 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
REFERENCE :
-
RCOCB8 Research Communications in Chemical Pathology and Pharmacology. (PJD Pub. Ltd., P.O. Box 966, Westbury, NY 11590) V.1- 1970- Volume(issue)/page/year: 13,723,1976
|
 CAS#:6785-55-3
CAS#:6785-55-3 CAS#:19121-58-5
CAS#:19121-58-5 CAS#:6106-33-8
CAS#:6106-33-8 CAS#:114696-99-0
CAS#:114696-99-0 CAS#:115388-90-4
CAS#:115388-90-4 CAS#:468-99-5
CAS#:468-99-5 CAS#:54302-48-6
CAS#:54302-48-6 CAS#:129850-55-1
CAS#:129850-55-1 CAS#:979-02-2
CAS#:979-02-2 CAS#:55320-51-9
CAS#:55320-51-9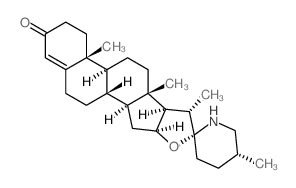 CAS#:17094-86-9
CAS#:17094-86-9
