Zamifenacin fumarate
Modify Date: 2024-01-11 19:17:10
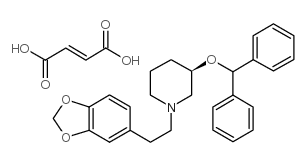
Zamifenacin fumarate structure
|
Common Name | Zamifenacin fumarate | ||
|---|---|---|---|---|
| CAS Number | 127308-98-9 | Molecular Weight | 531.59600 | |
| Density | N/A | Boiling Point | 700.2ºC at 760mmHg | |
| Molecular Formula | C31H33NO7 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 377.3ºC | |
Use of Zamifenacin fumarateZamifenacin fumarate (UK-76654 fumarate) is a potent gut-selective muscarinic M3 receptor antagonist. Zamifenacin significantly reduces colonic motility in irritable bowel syndrome[1]. |
| Name | (3R)-3-benzhydryloxy-1-[2-(1,3-benzodioxol-5-yl)ethyl]piperidine,but-2-enedioic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Zamifenacin fumarate (UK-76654 fumarate) is a potent gut-selective muscarinic M3 receptor antagonist. Zamifenacin significantly reduces colonic motility in irritable bowel syndrome[1]. |
|---|---|
| Related Catalog | |
| Target |
Muscarinic M3 receptor[1] |
| In Vivo | Zamifenacin exhibits moderate oral bioavailability (mouse 26%, rat 64%, dog 100%) and Cmax (mouse 92, rat 905, dog 416 ng/mL) following oral administration (mouse 13.2, rat 20 and, dog 5 mg/kg)[2]. Zamifenacin exhibits terminal elimination half-lives (mouse 2.1, rat 6.0 and, dog 1.1 h) due to high plasma clearance (68, 35, and 39 mL/min/kg respectively combined with large volumes of distribution (12.5, 19.0, and 3.5 L/kg respectively) following intravenous administration (mouse 5.3, rat 5.0 and, dog 1.0 mg/kg)[2]. Animal Model: Male CDl mice (mean weight 23 g)[2] Dosage: 5.3 mg/kg for i.v.; 13.2 mg/kg for oral (Pharmacokinetic Analysis) Administration: Intravenous administration and oral administration Result: Oral bioavailability (26%), Cmax (92 ng/mL), T1/2 (1.1 h). Animal Model: Male and female CD rats (mean weight 210 g)[2] Dosage: 5.0 mg/kg for i.v.; 20 mg/kg for oral (Pharmacokinetic Analysis) Administration: Intravenous administration and oral administration Result: Oral bioavailability (64%), Cmax (905 ng/mL), T1/2 (6.0 h). Animal Model: Male and two female beagle dogs (13-16 kg)[2] Dosage: 1.0 mg/kg for i.v.; 5 mg/kg for oral (Pharmacokinetic Analysis) Administration: Intravenous administration and oral administration Result: Oral bioavailability (100%), Cmax (416 ng/mL), T1/2 (1.1 h). |
| References |
| Boiling Point | 700.2ºC at 760mmHg |
|---|---|
| Molecular Formula | C31H33NO7 |
| Molecular Weight | 531.59600 |
| Flash Point | 377.3ºC |
| Exact Mass | 531.22600 |
| PSA | 105.53000 |
| LogP | 4.87810 |
| Storage condition | 2-8°C |
| zamifenacin fumarate |