Pregnanolone
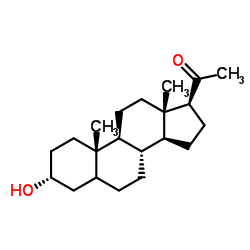
Pregnanolone structure
|
Common Name | Pregnanolone | ||
|---|---|---|---|---|
| CAS Number | 128-20-1 | Molecular Weight | 318.493 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 431.2±18.0 °C at 760 mmHg | |
| Molecular Formula | C21H34O2 | Melting Point | 149ºC | |
| MSDS | Chinese USA | Flash Point | 183.9±13.8 °C | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of PregnanolonePregnanolone, also known as 3a5b-Pregnanolone; Kabi-2213, SKF 6455, Eltanolone, is a cell membrane modulator potentially for the treatment of pain. Pregnanolone is an endogenous neurosteroid that is biosynthesized from progesterone. It is a positive allosteric modulator of the GABAA receptor, as well as a negative allosteric modulator of the glycine receptor, and is known to have sedative, anxiolytic, anesthetic, and anticonvulsant effects. It was investigated for clinical use as a general anesthetic, but produced unwanted side effects such as convulsions on occasion, and for that reason was never marketed. |
| Name | 3α-hydroxy-5β-pregnan-20-one |
|---|---|
| Synonym | More Synonyms |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 431.2±18.0 °C at 760 mmHg |
| Melting Point | 149ºC |
| Molecular Formula | C21H34O2 |
| Molecular Weight | 318.493 |
| Flash Point | 183.9±13.8 °C |
| Exact Mass | 318.255890 |
| PSA | 37.30000 |
| LogP | 4.89 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.524 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H351 |
| Precautionary Statements | P281 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R40 |
| Safety Phrases | 22-36 |
| RIDADR | NONH for all modes of transport |
| RTECS | TU4384000 |
| Precursor 8 | |
|---|---|
| DownStream 4 | |
|
A liquid chromatography-tandem mass spectrometry-based method for the simultaneous determination of hydroxy sterols and bile acids.
J. Chromatogr. A. 1371 , 184-95, (2014) Recently, hydroxy sterols and bile acids have gained growing interest as they are important regulators of energy homoeostasis and inflammation. The high number of different hydroxy sterols and bile ac... |
|
|
Glycine and GABA(A) ultra-sensitive ethanol receptors as novel tools for alcohol and brain research.
Mol. Pharmacol. 86(6) , 635-46, (2014) A critical obstacle to developing effective medications to prevent and/or treat alcohol use disorders is the lack of specific knowledge regarding the plethora of molecular targets and mechanisms under... |
|
|
Quantification of ten neuroactive steroids in plasma in Withdrawal Seizure-Prone and -Resistant mice during chronic ethanol withdrawal.
Psychopharmacol. Ser. 231(17) , 3401-14, (2014) The rapid membrane actions of neuroactive steroids, particularly via an enhancement of γ-aminobutyric acidA receptors (GABAARs), participate in the regulation of central nervous system excitability. P... |
| MFCD00067137 |
| Pregnanolone II |
| 5-Pregnan-3-ol-20-one |
| pregnanalone |
| Pregnan-20-one, 3-hydroxy-, (3α)- |
| PREGNAN-3A-OL-20-ONE |
| 3alpha-hydroxy-5beta-pregnan-20-one |
| (3α)-3-Hydroxypregnan-20-one |
| Pregnan-20-one,3-hydroxy-, (3a,5b)- |
| Butabindide oxalate |
 CAS#:128-23-4
CAS#:128-23-4 CAS#:110-54-3
CAS#:110-54-3 CAS#:57-83-0
CAS#:57-83-0 CAS#:80-92-2
CAS#:80-92-2 CAS#:108-24-7
CAS#:108-24-7 CAS#:91509-46-5
CAS#:91509-46-5 CAS#:55541-97-4
CAS#:55541-97-4 CAS#:81-25-4
CAS#:81-25-4 CAS#:80-89-7
CAS#:80-89-7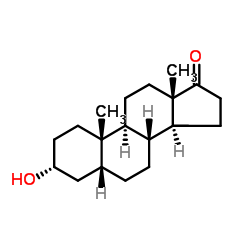 CAS#:53-42-9
CAS#:53-42-9