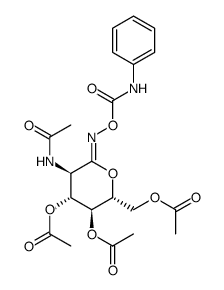
PugNAc
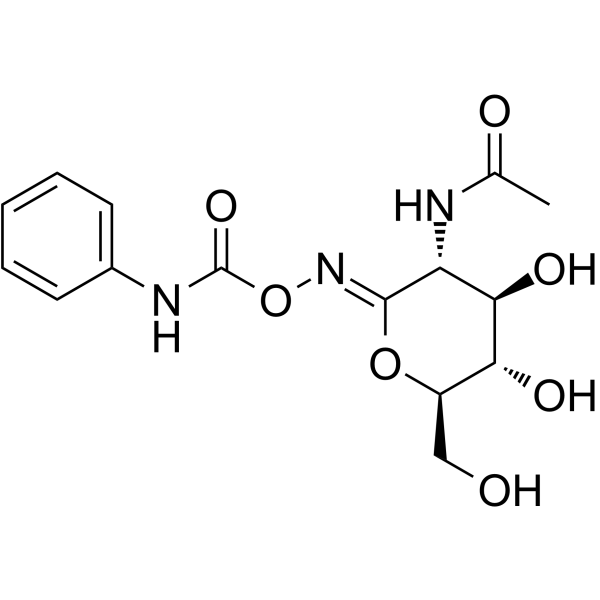
PugNAc structure
|
Common Name | PugNAc | ||
|---|---|---|---|---|
| CAS Number | 132489-69-1 | Molecular Weight | 353.33 | |
| Density | 1.53g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C15H19N3O7 | Melting Point | 172-175°C | |
| MSDS | USA | Flash Point | N/A | |
Use of PugNAc(Z)-PUGNAc is a potent O-GlcNAcase inhibitor. (Z)-PUGNAc is a vastly more potent inhibitor of O-GlcNAcase than the E form[1]. |
| Name | O-(2-Acetamido-2-deoxy-D-glucopyranosylidene)amino N-Phenylcarbamate |
|---|---|
| Synonym | More Synonyms |
| Description | (Z)-PUGNAc is a potent O-GlcNAcase inhibitor. (Z)-PUGNAc is a vastly more potent inhibitor of O-GlcNAcase than the E form[1]. |
|---|---|
| Related Catalog | |
| In Vitro | (Z)-PUGNAc amplifies the incorporation of O-GlcNAc on proteins within both HeLa and HEK cell systems[1]. |
| References |
| Density | 1.53g/cm3 |
|---|---|
| Melting Point | 172-175°C |
| Molecular Formula | C15H19N3O7 |
| Molecular Weight | 353.33 |
| Exact Mass | 353.12200 |
| PSA | 149.71000 |
| Index of Refraction | 1.638 |
| Storage condition | -20℃ |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
|
~71% 
PugNAc CAS#:132489-69-1 |
| Literature: Mohan, Halasyam; Vasella, Andrea Helvetica Chimica Acta, 2000 , vol. 83, # 1 p. 114 - 118 |
|
~% 
PugNAc CAS#:132489-69-1 |
| Literature: Helvetica Chimica Acta, , vol. 83, # 1 p. 114 - 118 |
|
~% 
PugNAc CAS#:132489-69-1 |
| Literature: Helvetica Chimica Acta, , vol. 83, # 1 p. 114 - 118 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Carbamidomethylation Side Reactions May Lead to Glycan Misassignments in Glycopeptide Analysis.
Anal. Chem. 87 , 6297-302, (2015) Iodoacetamide is perhaps the most widely used reagent for the alkylation of free sulfhydryls in proteomic experiments. Here, we report that both incomplete derivatization of Cys side chains and overal... |
|
|
The Role of PTP1B O-GlcNAcylation in Hepatic Insulin Resistance.
Int. J. Mol. Sci. 16 , 22856-69, (2015) Protein tyrosine phosphatase 1B (PTP1B), which can directly dephosphorylate both the insulin receptor and insulin receptor substrate 1 (IRS-1), thereby terminating insulin signaling, reportedly plays ... |
|
|
Glucosamine-induced Sp1 O-GlcNAcylation ameliorates hypoxia-induced SGLT dysfunction in primary cultured renal proximal tubule cells.
J. Cell Physiol. 229(10) , 1557-68, (2014) The aim of this study is to determine whether GlcN could recover the endoplasmic reticulum (ER) stress-induced dysfunction of Na(+) /glucose cotransporter (SGLT) in renal proximal tubule cells (PTCs) ... |
| (Z)-Pugnac,O-(2-Acetamido-2-deoxy-D-glucopyranosylidene)amino-Z-N-phenylcarbamate |
| O-(2-Acetamido-2-deoxy-D-glucopyranosylidenamino) N-phenylcarbamate |