Curdione
Modify Date: 2024-01-02 15:22:18
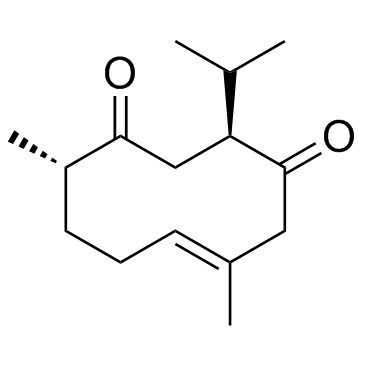
Curdione structure
|
Common Name | Curdione | ||
|---|---|---|---|---|
| CAS Number | 13657-68-6 | Molecular Weight | 236.350 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 347.6±42.0 °C at 760 mmHg | |
| Molecular Formula | C15H24O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 130.4±24.9 °C | |
Use of CurdioneCurdione, one of the major sesquiterpene compounds from Rhizoma Curcumae, has been shown to exhibit multiple bioactive properties.IC50 value: 60–80 μMTarget:In vitro: The study of the influence of curdione on the hemorheological changes in blood stasis model rats and thrombolysis in vitro showed that curdione only possessed thrombolytic effect in dose of 0.235 g·L-1 and 2.35 g·L-1, but has not the notable activity of thrombolysis [1]. The effects of curdione on human platelet aggregation induced by thrombin (0.3 U/ml) were tested in vitro. Curdione preferentially inhibited PAF- and thrombin- induced platelet aggregation in a concentration-dependent manner (IC50: 60–80 μM), whereas much higher concentrations of curdione were required to inhibit platelet aggregation induced by ADP and AA. Curdione also inhibited P-selectin expression in PAF-activated platelets. Moreover, curdione caused an increase in cAMP levels and attenuated intracellular Ca2+ mobilization in PAF-activated platelets. In vivo: Curdione showed significant antithrombotic activity [2]. |
| Name | (3S,6E,10S)-6,10-dimethyl-3-propan-2-ylcyclodec-6-ene-1,4-dione |
|---|---|
| Synonym | More Synonyms |
| Description | Curdione, one of the major sesquiterpene compounds from Rhizoma Curcumae, has been shown to exhibit multiple bioactive properties.IC50 value: 60–80 μMTarget:In vitro: The study of the influence of curdione on the hemorheological changes in blood stasis model rats and thrombolysis in vitro showed that curdione only possessed thrombolytic effect in dose of 0.235 g·L-1 and 2.35 g·L-1, but has not the notable activity of thrombolysis [1]. The effects of curdione on human platelet aggregation induced by thrombin (0.3 U/ml) were tested in vitro. Curdione preferentially inhibited PAF- and thrombin- induced platelet aggregation in a concentration-dependent manner (IC50: 60–80 μM), whereas much higher concentrations of curdione were required to inhibit platelet aggregation induced by ADP and AA. Curdione also inhibited P-selectin expression in PAF-activated platelets. Moreover, curdione caused an increase in cAMP levels and attenuated intracellular Ca2+ mobilization in PAF-activated platelets. In vivo: Curdione showed significant antithrombotic activity [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 0.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 347.6±42.0 °C at 760 mmHg |
| Molecular Formula | C15H24O2 |
| Molecular Weight | 236.350 |
| Flash Point | 130.4±24.9 °C |
| Exact Mass | 236.177628 |
| PSA | 34.14000 |
| LogP | 3.12 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.458 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 29143990 |
|---|
| (3R,6E,10S)-3-Isopropyl-6,10-dimethyl-6-cyclodecene-1,4-dione |
| N1432 |
| Curdione |
| 6-Cyclodecene-1,4-dione, 6,10-dimethyl-3-(1-methylethyl)-, (3R,6E,10S)- |
| neo-Curdione |
| Germacr-1(10)-ene-5,8-dione |
| (3S,10S)-3-Isopropyl-6,10-dimethyl-6-cyclodecene-1,4-dione |
| 6-Cyclodecene-1,4-dione, 6,10-dimethyl-3-(1-methylethyl)-, (3S,10S)- |

