N3PT
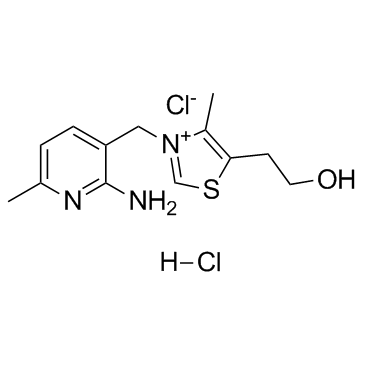
N3PT structure
|
Common Name | N3PT | ||
|---|---|---|---|---|
| CAS Number | 13860-66-7 | Molecular Weight | 336.28000 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C13H19Cl2N3OS | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of N3PTN3PT(N3-pyridyl thiamine) is a potent and selective transketolase(TK) inhibitor (IC50= 22 nM for Apo-TK) both in vitro and in vivo.IC50 Value: 22 nM( Apo-TK) ; 26 nM (EC50, Cellular TK) [1]Target: transketolasein vitro: N3PT inhibits transketolase activity in a cell based assay. Competitive inhibition of TK by N3PT in cells treated with increasing doses of thiamine, expressed as percentage enzymatic activity (the slope of initial linear range) of controls not treated with compounds [1].in vivo: Tumors were induced in mice at day 0 and mice were then treated at day 7 with vehicle alone or with N3PT [2]. Low-thiamine diet enhances the sensitivity to N3PT inhibition of TK in spleen. Animals were switched to diets containing 16.5 mg/kg (unchanged), 5 mg/kg, 1 mg/kg, or 0 mg/kg thiamine, from a normal chow containing 16.5mg/kg thiamine [1]. |
| Name | 3-[(2-Amino-6-methyl-3-pyridinyl)methyl]-5-(2-hydroxyethyl)-4-met hyl-1,3-thiazol-3-ium chloride hydrochloride (1:1:1) |
|---|---|
| Synonym | More Synonyms |
| Description | N3PT(N3-pyridyl thiamine) is a potent and selective transketolase(TK) inhibitor (IC50= 22 nM for Apo-TK) both in vitro and in vivo.IC50 Value: 22 nM( Apo-TK) ; 26 nM (EC50, Cellular TK) [1]Target: transketolasein vitro: N3PT inhibits transketolase activity in a cell based assay. Competitive inhibition of TK by N3PT in cells treated with increasing doses of thiamine, expressed as percentage enzymatic activity (the slope of initial linear range) of controls not treated with compounds [1].in vivo: Tumors were induced in mice at day 0 and mice were then treated at day 7 with vehicle alone or with N3PT [2]. Low-thiamine diet enhances the sensitivity to N3PT inhibition of TK in spleen. Animals were switched to diets containing 16.5 mg/kg (unchanged), 5 mg/kg, 1 mg/kg, or 0 mg/kg thiamine, from a normal chow containing 16.5mg/kg thiamine [1]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C13H19Cl2N3OS |
|---|---|
| Molecular Weight | 336.28000 |
| Exact Mass | 335.06300 |
| PSA | 91.26000 |
| Storage condition | 2-8℃ |
|
~% 
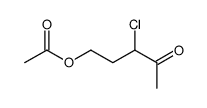
N3PT CAS#:13860-66-7 |
| Literature: Matsukawa; Matsuno Yakugaku Zasshi, 1944 , vol. 64, # 3 p. 145,150 Chem.Abstr., 1951 , p. 4724 |
|
~% 
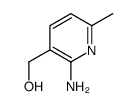
N3PT CAS#:13860-66-7 |
| Literature: Dornow; Hargesheimer Chemische Berichte, 1953 , vol. 86, p. 461,464 |
|
~% 
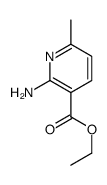
N3PT CAS#:13860-66-7 |
| Literature: Dornow; Hargesheimer Chemische Berichte, 1953 , vol. 86, p. 461,464 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| 3-(2-amino-6-methyl-pyridin-3-ylmethyl)-5-(2-hydroxy-ethyl)-4-methyl-thiazolium,chloride hydrochloride |
| 2-(2H-pyrazol-3-yl)-5-(trifluoromethyl)aniline |
| 3-(2-Amino-6-methyl-[3]pyridylmethyl)-5-(2-hydroxy-aethyl)-4-methyl-thiazolium,Chlorid-hydrochlorid |
| 3-(2-amino-6-methyl-pyridin-3-ylmethyl)-5-(2-hydroxy-ethyl)-4-methyl-thiazol-3-ium chloride hydrochloride |
| 2-(1(2)H-pyrazol-3-yl)-5-trifluoromethyl-aniline |
| 3-(2-Amino-4-trifluormethylphenyl)pyrazol |
| 3-(2-amino-6-methyl-[3]pyridylmethyl)-5-(2-hydroxy-ethyl)-4-methyl-thiazolium,chloride-hydrochloride |
| N3PT |