O-SUCCINYL-L-HOMOSERINE
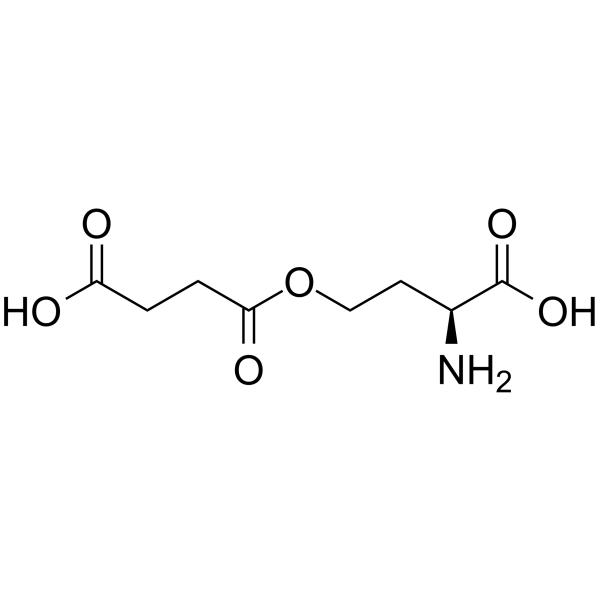
O-SUCCINYL-L-HOMOSERINE structure
|
Common Name | O-SUCCINYL-L-HOMOSERINE | ||
|---|---|---|---|---|
| CAS Number | 1492-23-5 | Molecular Weight | 219.19200 | |
| Density | 1.392g/cm3 | Boiling Point | 492.8ºC at 760mmHg | |
| Molecular Formula | C8H13NO6 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 251.8ºC | |
Use of O-SUCCINYL-L-HOMOSERINEO-Succinyl-L-homoserine is a homoserine derivative. O-Succinyl-L-homoserine is an intermediate in the biosynthesis of methionine in Escherichia coli and Salmonella typhimurium[1]. |
| Name | O-succinyl-L-homoserine |
|---|---|
| Synonym | More Synonyms |
| Description | O-Succinyl-L-homoserine is a homoserine derivative. O-Succinyl-L-homoserine is an intermediate in the biosynthesis of methionine in Escherichia coli and Salmonella typhimurium[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.392g/cm3 |
|---|---|
| Boiling Point | 492.8ºC at 760mmHg |
| Molecular Formula | C8H13NO6 |
| Molecular Weight | 219.19200 |
| Flash Point | 251.8ºC |
| Exact Mass | 219.07400 |
| PSA | 126.92000 |
| Vapour Pressure | 4.68E-11mmHg at 25°C |
| Index of Refraction | 1.515 |
| Storage condition | -20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2922509090 |
| Precursor 0 | |
|---|---|
| DownStream 2 | |
| HS Code | 2922509090 |
|---|---|
| Summary | 2922509090. other amino-alcohol-phenols, amino-acid-phenols and other amino-compounds with oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
The enzymology of cystathionine biosynthesis: strategies for the control of substrate and reaction specificity.
Arch. Biochem. Biophys. 433 , 166-175, (2005) The ability of enzymes to catalyze specific reactions, while excluding others, is central to cellular metabolism. Control of reaction specificity is of particular importance for enzymes that employ ca... |
|
|
Cloning and characterization of two Lactobacillus casei genes encoding a cystathionine lyase.
Appl. Environ. Microbiol. 74 , 99-106, (2008) Volatile sulfur compounds are key flavor compounds in several cheese types. To better understand the metabolism of sulfur-containing amino acids, which certainly plays a key role in the release of vol... |
|
|
Enzymatic characterization and inhibitor discovery of a new cystathionine {gamma}-synthase from Helicobacter pylori.
J. Biochem. 143 , 59-68, (2008) Cystathionine gamma-synthase (CGS) catalyses the first step of the transsulfuration pathway that converts l-cysteine to l-homocysteine in bacteria, whereas this pathway is absent in human. In this rep... |
| (2S)-2-amino-4-(3-carboxypropanoyloxy)butanoic acid |
| O-Succinyl-L-homoserine |
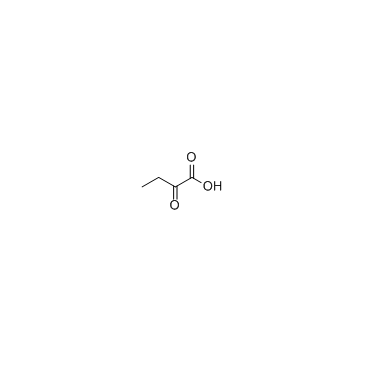 CAS#:600-18-0
CAS#:600-18-0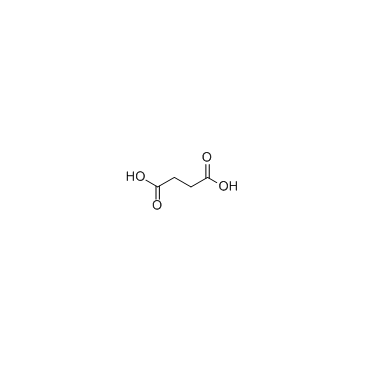 CAS#:110-15-6
CAS#:110-15-6