Poncirin
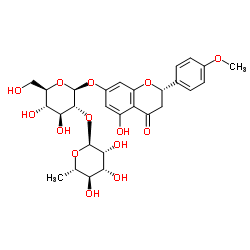
Poncirin structure
|
Common Name | Poncirin | ||
|---|---|---|---|---|
| CAS Number | 14941-08-3 | Molecular Weight | 594.561 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 900.1±65.0 °C at 760 mmHg | |
| Molecular Formula | C28H34O14 | Melting Point | 210ºC | |
| MSDS | Chinese USA | Flash Point | 296.5±27.8 °C | |
Use of PoncirinPoncirin is isolated from Poncirus trifoliata with anti-inflammory activites. Poncirin significantly reduces mechanical hyperalgesia and allodynia in Complete Freund’s Adjuvant (CFA)-induced inflammatory pain models[1]. |
| Name | (2S)-poncirin |
|---|---|
| Synonym | More Synonyms |
| Description | Poncirin is isolated from Poncirus trifoliata with anti-inflammory activites. Poncirin significantly reduces mechanical hyperalgesia and allodynia in Complete Freund’s Adjuvant (CFA)-induced inflammatory pain models[1]. |
|---|---|
| Related Catalog | |
| In Vivo | Poncirin (intraperitoneal administration; 30 mg/kg) markedly reduces the pain behavior in both acetic acid-induced visceral pain and formalin-induced tonic pain models inComplete Freund’s Adjuvant (CFA)-induced inflammatory pain model[1]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 900.1±65.0 °C at 760 mmHg |
| Melting Point | 210ºC |
| Molecular Formula | C28H34O14 |
| Molecular Weight | 594.561 |
| Flash Point | 296.5±27.8 °C |
| Exact Mass | 594.194885 |
| PSA | 214.06000 |
| LogP | 3.39 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.678 |
| Safety Phrases | 22-24/25 |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
~% 
Poncirin CAS#:14941-08-3 |
| Literature: Chen, Lao-Jer; Hrazdina, Geza Phytochemistry (Elsevier), 1981 , vol. 20, p. 297 - 304 |
|
~% 
Poncirin CAS#:14941-08-3 |
| Literature: Phytochemistry (Elsevier), , vol. 20, p. 297 - 304 |
|
~% 
Poncirin CAS#:14941-08-3 |
| Literature: Phytochemistry (Elsevier), , vol. 20, p. 297 - 304 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| (2S)-7-{[(2S,3R,4S,5S,6R)-4,5-Dihydroxy-6-(hydroxymethyl)-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}tetrahydro-2H-pyran-2-yl]oxy}-5-hydroxy-2-(4-methoxyphenyl)-2,3-dihydro-4H-chromen-4-one |
| Einecs 239-020-1 |
| Neohesperidoside isosakuranetin-7 |
| 4H-1-Benzopyran-4-one, 7-[[2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-2,3-dihydro-5-hydroxy-2-(4-methoxyphenyl)-, (2S)- |
| Poncirin |
| (2S)-5-Hydroxy-2-(4-methoxyphenyl)-4-oxo-3,4-dihydro-2H-chromen-7-yl-2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranoside |
| (2S)-7-{[(2S,3R,4S,5S,6R)-4,5-Dihydroxy-6-(hydroxyméthyl)-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-méthyltétrahydro-2H-pyran-2-yl]oxy}tétrahydro-2H-pyran-2-yl]oxy}-5-hydroxy-2-(4-méthoxyphényl)-2,3-dihydro-4H-chromén-4-one |
| Isosakuranetin 7-O-neohesperidoside |
| (S)-7-((2-O-(6-Deoxy-a-L-mannopyranosyl)-b-D-glucopyranosyl)oxy)-2,3-dihydro-5-hydroxy-2-(4-methoxyphenyl)-4H-benzopyran-4-one |
| ISOSAKURANETIN-7-NEOHESPERIDOSIDE |
| (2S)-7-{[(2S,3R,4S,5S,6R)-4,5-Dihydroxy-6-(hydroxymethyl)-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}tetrahydro-2H-pyran-2-yl]oxy}-5-hydroxy-2-(4-methoxyphenyl)-2,3-dihydro-4H-chromen-4-on |
| (S)-7-((2-O-(6-Deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl)oxy)-2,3-dihydro-5-hydroxy-2-(4-methoxyphenyl)-4H-benzopyran-4-one |
| 4'-O-Methylnaringin |
| ISOSAKURANETIN-7-O-NEOHESPERIDOSID |
| (2S)-5-Hydroxy-2-(4-methoxyphenyl)-4-oxo-3,4-dihydro-2H-chromen-7-yl 2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranoside |
| Isosakuranetin-7-O-neohesperidoside |
| Citrifolioside |