Emivirine
Modify Date: 2024-01-01 22:32:29
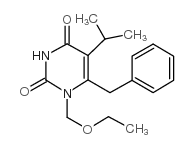
Emivirine structure
|
Common Name | Emivirine | ||
|---|---|---|---|---|
| CAS Number | 149950-60-7 | Molecular Weight | 302.37 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C17H22N2O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of EmivirineEmivirine (MKC-442) is a non-nucleoside reverse transcriptase inhibitors (NNRTIs) with Ki values of 0.20 and 0.01 μM for dTTP- and dGTP-dependent DNA or RNA polymerase activity, respectively. Emivirine displays potent and selective anti-human immunodeficiency virus type 1 (HIV-1) activity[1][2]. |
| Name | emivirine |
|---|---|
| Synonym | More Synonyms |
| Description | Emivirine (MKC-442) is a non-nucleoside reverse transcriptase inhibitors (NNRTIs) with Ki values of 0.20 and 0.01 μM for dTTP- and dGTP-dependent DNA or RNA polymerase activity, respectively. Emivirine displays potent and selective anti-human immunodeficiency virus type 1 (HIV-1) activity[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Emivirine (EMV) is also specific for HIV-1 RT and was without effect on HIV-2[2]. Emivirine (EMV) has no obvious toxicity for human healthy cells[2]. Cell Viability Assay[2] Cell Line: Human bone marrow cells collected from normal healthy volunteers. Concentration: 0, 0.1, 1, 10, or 100 μM. Incubation Time: 14 days. Result: At concentrations of 0.1 to 10 μM, no effect on cell growth, lactic acid production, mitochondrial DNA synthesis, or mitochondrial structure was seen compared to what occurred with untreated HepG2 cells. |
| In Vivo | Tthe approximate lethal oral dose of Emivirine (EMV) for rats was ≥3 g/kg for males and 2.5 g/kg for females[2]. Animal Model: Male Sprague-Dawley rats[2]. Dosage: 50 mg/kg. Administration: Gavage. Result: The oral absorption was 68%. |
| References |
| Molecular Formula | C17H22N2O3 |
|---|---|
| Molecular Weight | 302.37 |
| Exact Mass | 302.16300 |
| PSA | 64.09000 |
| LogP | 2.24480 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2933990090 |
|---|
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| I-EBU |
| 1EtOMe6Bz5i-Pr-U |
| Emivirine |
| Emivirine [USAN:INN] |
| [14C]-Emivirine |
| 1-(ethoxymethyl)-5-(1-methylethyl)-6-(phenylmethyl)pyrimidine-2,4(1H,3H)-dione |
| Coactinon |
| Mkc 442 |
| 1-ethoxymethyl-5-isopropyl-6-benzyluracil |
| 6-benzyl-1-(ethoxymethyl)-5-isopropyl uracil |
| 6-benzyl-1-(ethoxymethyl)-5-propan-2-ylpyrimidine-2,4-dione |
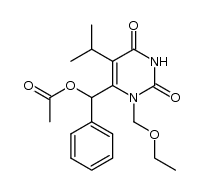 CAS#:161061-33-2
CAS#:161061-33-2 CAS#:176519-55-4
CAS#:176519-55-4 CAS#:3188-13-4
CAS#:3188-13-4 CAS#:462-95-3
CAS#:462-95-3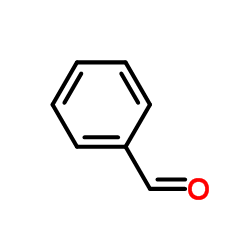 CAS#:100-52-7
CAS#:100-52-7 CAS#:1026065-34-8
CAS#:1026065-34-8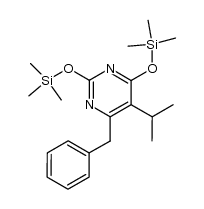 CAS#:1020069-04-8
CAS#:1020069-04-8 CAS#:176519-53-2
CAS#:176519-53-2