Abiraterone Acetate
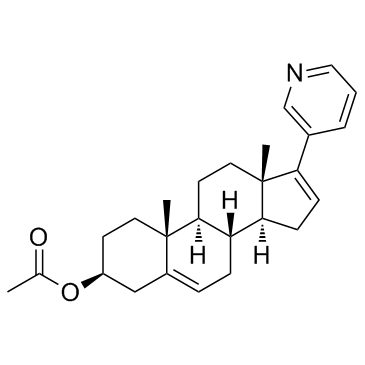
Abiraterone Acetate structure
|
Common Name | Abiraterone Acetate | ||
|---|---|---|---|---|
| CAS Number | 154229-18-2 | Molecular Weight | 391.546 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 506.7±50.0 °C at 760 mmHg | |
| Molecular Formula | C26H33NO2 | Melting Point | 127-130°C | |
| MSDS | Chinese USA | Flash Point | 260.2±30.1 °C | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of Abiraterone AcetateAbiraterone acetate is an oral, potent, selective, and irreversible inhibitor of CYP17. |
| Name | abiraterone acetate |
|---|---|
| Synonym | More Synonyms |
| Description | Abiraterone acetate is an oral, potent, selective, and irreversible inhibitor of CYP17. |
|---|---|
| Related Catalog | |
| Target |
CYP17[1] |
| In Vitro | Abiraterone (Abi) acetate is an ester prodrug of the anticancer agent Abiraterone, which shows IC50 values of 15 nM and 2.5 nM for the 17,20-lyase and 17α-hydroxylase (CYP17 is a bifunctional enzyme with both 17α-hydroxylase and 17,20-lyase activity). Abiraterone inhibits human 17,20-lyase and 17α-hydroxylase with IC50 of 27 and 30 nM respectively[1]. Significant inhibition of proliferation of the AR-positive prostate cancer cell lines LNCaP and VCaP with doses of Abiraterone ≥5 μM is confirmed[2]. Abiraterone inhibits recombinant human 3βHSD1 and 3βHSD2 activity with competitive Ki values of 2.1 and 8.8 μM. 10 μM Abiraterone is sufficient to completely block synthesis of 5α-dione and DHT in both cell lines.Treatment with Abiraterone significantly inhibited CRPC progression in the robustly growing subset, effectively putting a ceiling on tumor growth over 4 weeks of treatment (P<0.00001)[3]. |
| In Vivo | Abiraterone (Abi) acetate prolongs survival in castration-resistant prostate cancer (CRPC). [3H]-dehydroepiandrosterone (DHEA) depletion and Δ4-androstenedione (AD) accumulation are inhibited by Abiraterone in LNCaP, with an IC50<1 μM. The 0.5 mmol/kg/d Abiraterone treatment dose is previously shown to yield serum concentrations of about 0.5 to 1 μM. Xenograft tumor growth in the control group is widely variable, with some tumors growing slowly and only a subset of tumors exhibiting robust growth[3]. |
| Cell Assay | LNCaP and VCaP cells are seeded in 96-well plates and grown in CSS-supplemented phenol red-free or FBS-supplemented media for 7 days. Cells are treated with Abiraterone (5 μM and 10 μM) at 24 and 96 hours after plating and cell viability is determined on day 7 by adding CellTiter Glo and measuring luminescence[2]. |
| Animal Admin | Mice[3] Male NOD/SCID mice 6 to 8 weeks of age are surgically orchiectomized and implanted with a 5 mg 90-day sustained release DHEA pellet to mimic CRPC with human adrenal physiology. Two days later, 7×106 LAPC4 cells are injected subcutaneously with Matrigel. Tumor dimensions are measured 2 to 3 times per week, and volume is calculated as length×width×height×0.52. Once tumors reach 300 mm3, mice are randomly assigned to vehicle or Abiraterone treatment groups. Mice in the Abiraterone group are treated with 5 mL/kg intraperitoneal injections of 0.5 mmol/kg/d (0.1 mL 5% benzyl alcohol and 95% safflower oil solution) and control mice with vehicle only, once daily for 5 days per week over a duration of 4 weeks (n=8 mice per treatment). Statistical significance between Abiraterone and vehicle treatment groups is assessed by ANOVA based on a mixed-effect model. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 506.7±50.0 °C at 760 mmHg |
| Melting Point | 127-130°C |
| Molecular Formula | C26H33NO2 |
| Molecular Weight | 391.546 |
| Flash Point | 260.2±30.1 °C |
| Exact Mass | 391.251129 |
| PSA | 39.19000 |
| LogP | 6.55 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.584 |
| Storage condition | -20°C Freezer |
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H360-H372 |
| Precautionary Statements | P201-P260-P280-P308 + P313 |
| Target Organs | Endocrine system |
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| RTECS | BV7992100 |
| HS Code | 2933399090 |
| Precursor 10 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy.
J. Clin. Oncol. 28(9) , 1481-8, (2010) Abiraterone acetate is a prodrug of abiraterone, a selective inhibitor of CYP17, the enzyme catalyst for two essential steps in androgen biosynthesis. In castration-resistant prostate cancers (CRPCs),... |
|
|
Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate.
J. Clin. Oncol. 28(9) , 1489-95, (2010) The principal objective of this trial was to evaluate the antitumor activity of abiraterone acetate, an oral, specific, irreversible inhibitor of CYP17 in docetaxel-treated patients with castration-re... |
|
|
Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer.
J. Clin. Oncol. 28(9) , 1496-501, (2010) Persistence of ligand-mediated androgen receptor signaling has been documented in castration-resistant prostate cancers (CRPCs). Abiraterone acetate (AA) is a potent and selective inhibitor of CYP17, ... |
| 17-(3-pyridyl)-5,16-androstadien-3beta-acetate |
| Abiraterone acetate |
| Androsta-5,16-dien-3-ol, 17-(3-pyridinyl)-, acetate (ester), (3β)- |
| Zytiga |
| (3β)-17-(3-Pyridinyl)androsta-5,16-dien-3-yl acetate |
| (3β)-17-(pyridin-3-yl)androsta-5,16-dien-3-yl acetate |
| Abiraterone (acetate) |
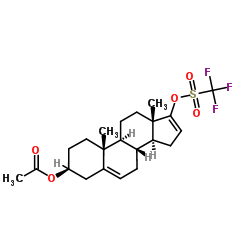 CAS#:115375-60-5
CAS#:115375-60-5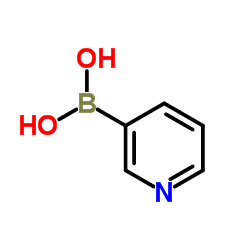 CAS#:1692-25-7
CAS#:1692-25-7 CAS#:108-24-7
CAS#:108-24-7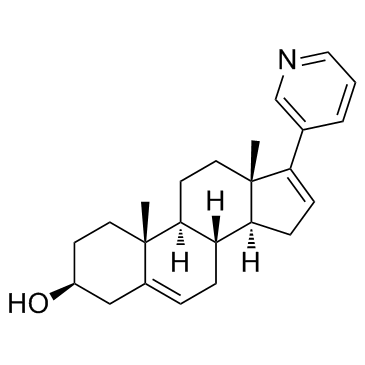 CAS#:154229-19-3
CAS#:154229-19-3 CAS#:75-36-5
CAS#:75-36-5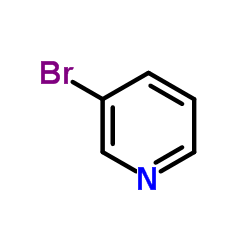 CAS#:626-55-1
CAS#:626-55-1![[10,13-dimethyl-17-[(4-methylphenyl)sulfonylhydrazinylidene]-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-3-yl] acetate Structure](https://image.chemsrc.com/caspic/478/89359-48-8.png) CAS#:89359-48-8
CAS#:89359-48-8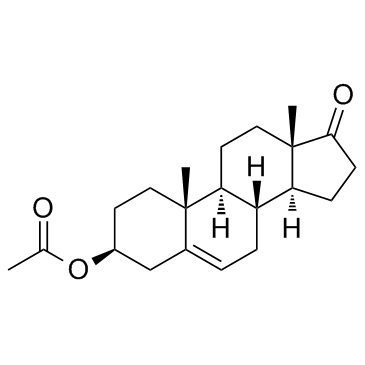 CAS#:853-23-6
CAS#:853-23-6 CAS#:89878-14-8
CAS#:89878-14-8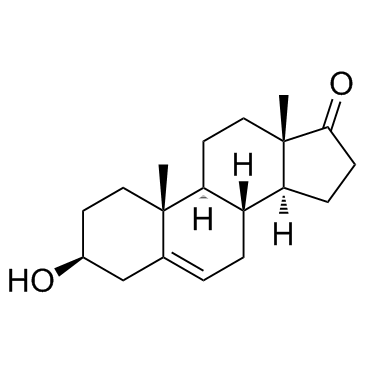 CAS#:53-43-0
CAS#:53-43-0
