Cariporide
Modify Date: 2024-01-03 10:41:28
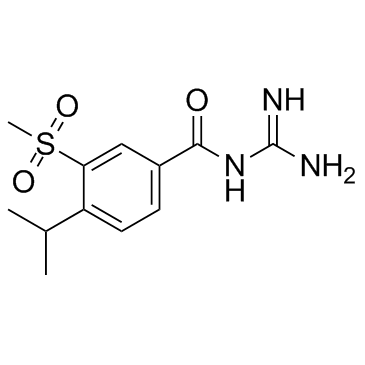
Cariporide structure
|
Common Name | Cariporide | ||
|---|---|---|---|---|
| CAS Number | 159138-80-4 | Molecular Weight | 283.347 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 542.8±60.0 °C at 760 mmHg | |
| Molecular Formula | C12H17N3O3S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 282.1±32.9 °C | |
Use of CariporideCariporide (HOE-642) is a selective Na+/H+ exchange inhibitor. |
| Name | N-(diaminomethylidene)-3-methylsulfonyl-4-propan-2-ylbenzamide |
|---|---|
| Synonym | More Synonyms |
| Description | Cariporide (HOE-642) is a selective Na+/H+ exchange inhibitor. |
|---|---|
| Related Catalog | |
| In Vitro | Cariporide significantly suppresses markers of cell death, such as TUNEL positivity and caspase-3 cleavage, at 8 or 16 hours. Cariporide remarkably suppresses cytosolic Na+ and Ca2+ accumulation. Cariporide prevents mitochondrial membrane potential loss induced by H2+O2+[1]. Cariporide (HOE-642) ameliorates myocardial ischemia/reperfusion injury, by the well-established reduction of cytosolic Ca2+ in cardiac myocytes through inhibition of Na+/H+ exchange[2]. Cariporide (HOE-642), has inhibitory effects on the degranulation of human platelets, the formation of platelet–leukocyte-aggregates, and the activation of the GPIIb/IIIa receptor (PAC-1)[3]. |
| In Vivo | Intravenous administration of cariporide significantly decreases brain Na+ uptake and reduces cerebral edema, brain swelling, and infarct volume[4]. |
| Cell Assay | Neonatal rat cardiomyocytes are randomly separated into groups: (1) control group, (2) incubation with 100 μM hydrogen peroxide, or (3) pretreatment with 10 μM cariporide for 20 minutes followed by 100 μM hydrogen peroxide. Caspase-3 activity is measured by detection of the cleavage of a colorimetric caspase-3 substrate, N-acetyl-Asp-Glu-Val-Asp-p-nitroaniline, using an assay kit[1]. |
| Animal Admin | Rats: Cariporide and/or bumetanide are administered intravenously (15 or 30 mg/kg in 2 to 4 doses, respectively, of 7.5 mg/kg) starting at 20 minutes before initiation of pMCAO. For neurologic outcome experiments, some rats are given cariporide and/or bumetanide by a single intraperitoneal injection[4]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 542.8±60.0 °C at 760 mmHg |
| Molecular Formula | C12H17N3O3S |
| Molecular Weight | 283.347 |
| Flash Point | 282.1±32.9 °C |
| Exact Mass | 283.099060 |
| PSA | 123.99000 |
| LogP | 0.40 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.602 |
| Storage condition | 2-8℃ |
| RIDADR | NONH for all modes of transport |
|---|---|
| RTECS | CV2310560 |
|
~91% 
Cariporide CAS#:159138-80-4 |
| Literature: Weichert, Andreas; Faber, Sabine; Jansen, Hans W.; Scholz, Wolfgang; Lang, Hans J. Arzneimittel-Forschung/Drug Research, 1997 , vol. 47, # 11 p. 1204 - 1207 |
|
~% 
Cariporide CAS#:159138-80-4 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 47, # 11 p. 1204 - 1207 |
| HOE642 |
| Cariporide |
| N-(Diaminomethylene)-4-isopropyl-3-(methylsulfonyl)benzamide |
| Benzamide, N-(diaminomethylene)-4-(1-methylethyl)-3-(methylsulfonyl)- |
| N-Carbamimidoyl-4-isopropyl-3-(methylsulfonyl)benzamide |
| benzamide, N-(aminoiminomethyl)-4-(1-methylethyl)-3-(methylsulfonyl)- |
| N-(diaminomethylidene)-3-(methylsulfonyl)-4-(propan-2-yl)benzamide |