MK 0677
Modify Date: 2024-01-01 18:36:29
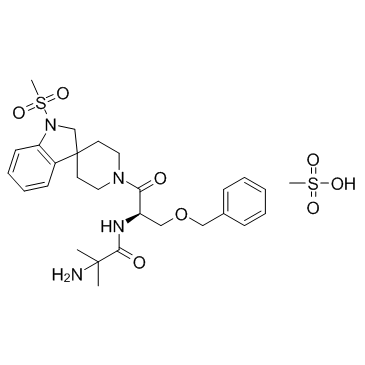
MK 0677 structure
|
Common Name | MK 0677 | ||
|---|---|---|---|---|
| CAS Number | 159752-10-0 | Molecular Weight | 624.769 | |
| Density | N/A | Boiling Point | 868.9ºC at 760 mmHg | |
| Molecular Formula | C28H40N4O8S2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 479.3ºC | |
Use of MK 0677Ibutamoren (Mesylate) is a potent, non-peptide Growth hormone secretagogue receptor (GHSR) agonist. |
| Name | 2-amino-2-methyl-N-[(2R)-1-(1-methylsulfonylspiro[2H-indole-3,4'-piperidine]-1'-yl)-1-oxo-3-phenylmethoxypropan-2-yl]propanamide,methanesulfonic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Ibutamoren (Mesylate) is a potent, non-peptide Growth hormone secretagogue receptor (GHSR) agonist. |
|---|---|
| Related Catalog | |
| In Vivo | Ibutamoren mesylate (5 mg/kg/day) results in a statistically significant increases body weight gain and increases serum IGF-1 and GH levels in dogs. Ibutamoren mesylate results in no significant increase in CSF IGF-1 or GH levels on days 7 or 15 of the study[1]. Pretreating mice with GH blocks activation of these neurons by Ibutamoren mesylate (50 μg, i.p.). In the knockout mice, both GH and octreotide fail to inhibit Ibutamoren mesylate activation of arcuate neurons[2]. Chronic oral administration of MK-0677 is associated with significant increases in GH and IGF-I levels that are maintained for the duration of the treatment. The GH profile following MK-0677 administration consists of episodic increases above control[3]. MK-0677 significantly increases peak GH concentrations after oral administration. MK-0677 is a potent GH secretagogue that induces an immediate, large, long lasting increase in GH levels when administered orally or i.v[4]. |
| Animal Admin | Compounds used are: Ibutamoren mesylate (50 μg), octreotide (100 μg), and mGH (30 μg). Mice are give an initial ip injection (0.1 mL) of either saline, octreotide or mGH, followed 10 min later by an ip injection (0.1 mL) of either saline or Ibutamoren mesylate. Thus, the first study comprised of the following groups: saline/saline, saline/Ibutamoren mesylate, mGH/saline, mGH/Ibutamoren mesylate saline/saline, saline/Ibutamoren mesylate, mGH/saline, mGH/Ibutamoren mesylate, and the second study of: saline/saline, saline/Ibutamoren mesylate, octreotide/saline, octreotide/Ibutamoren mesylate. Additionally, a number of mice are injected ip with hypertonic saline (0.2 mL, 1.5 M) to serve as positive controls for the immunocytochemistry. Ninety minutes after injection animals are terminally anesthetized with sodium pentobarbitone (60 mg/kg, ip) and perfused transcardially with heparinized saline followed by 4% paraformaldehyde in 0.1mol/Lphosphate buffer (PB, pH 7.4). Brains are then removed and placed in the same solution for 24 h before being stored at− 80°C until processing. Coronal sections of forebrain (40 μM) are cut on a freezing microtome and placed in 0.1mol/LPB containing Triton X-100 (PB-T, pH 7.4). Sections are incubated for 24 h at 4°C in Ab-2 Fos antibody (rabbit polyclonal; 1:1000 in 1% normal sheep serum). The antibody-antigen complex is localized with a 1-h incubation in biotinylated anti-rabbit Ig, followed by a 1-h incubation in avidin, biotinylated horseradish peroxidase. The reaction product is visualized using a glucose oxidase-diaminobenzidine-nickel method, and Fos-like immunoreactivity is visualized as a dense purple-black precipitate restricted to the nucleus. The number of c-fos positive nuclei in the arcuate and periventricular nuclei (six to eight sections per mouse) are counted double-blind and a group mean calculated (mean±sem). Statistical analysis is performed by a two-way ANOVA followed by an all pairwise multiple comparison of data with significance taken as P < 0.05. |
| References |
| Boiling Point | 868.9ºC at 760 mmHg |
|---|---|
| Molecular Formula | C28H40N4O8S2 |
| Molecular Weight | 624.769 |
| Flash Point | 479.3ºC |
| Exact Mass | 624.228760 |
| PSA | 196.66000 |
| LogP | 4.97440 |
| Appearance of Characters | white to beige |
| Vapour Pressure | 3.05E-32mmHg at 25°C |
| Storage condition | 2-8°C |
| Water Solubility | H2O: soluble5mg/mL, clear |
| RIDADR | NONH for all modes of transport |
|---|---|
| RTECS | TX1404490 |
| Ibutamoren mesylate (USAN) |
| S1151_Selleck |
| Ibutamoren |
| Ibutamoren mesilate |
| Ibutamoren mesylate |
| Ibutamoren (Mesylate) |
| MK-0677 |
| MK-0667 |
| Propanamide, 2-amino-N-[(1R)-2-[1,2-dihydro-1-(methylsulfonyl)spiro[3H-indole-3,4'-piperidin]-1'-yl]-2-oxo-1-[(phenylmethoxy)methyl]ethyl]-2-methyl-, methanesulfonate (1:1) |
| MK-677 |
| 1'-(2-Methylalanyl-O-benzyl-D-seryl)-1-(methylsulfonyl)-1,2-dihydrospiro[indole-3,4'-piperidine] methanesulfonate (1:1) |
| Crescendo |
| UNII-R90JB6QJ2B |