Rofecoxib
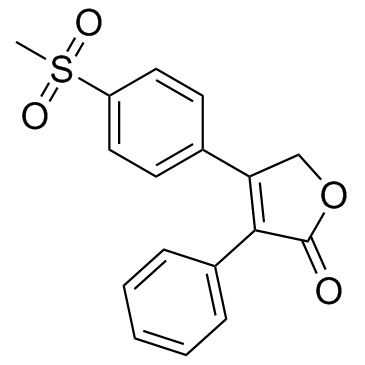
Rofecoxib structure
|
Common Name | Rofecoxib | ||
|---|---|---|---|---|
| CAS Number | 162011-90-7 | Molecular Weight | 314.356 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 577.6±50.0 °C at 760 mmHg | |
| Molecular Formula | C17H14O4S | Melting Point | 207°C | |
| MSDS | Chinese USA | Flash Point | 303.1±30.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of RofecoxibRofecoxib is a potent, specific and orally active COX-2 inhibitor, with IC50s of 26 and 18 nM for human COX-2 in human osteosarcoma cells and Chinese hamster ovary cells, with a 1000-fold selectivity for COX-2 over human COX-1 (IC50 > 50 μM in U937 cells and > 15 μM in Chinese hamster ovary cells). |
| Name | rofecoxib |
|---|---|
| Synonym | More Synonyms |
| Description | Rofecoxib is a potent, specific and orally active COX-2 inhibitor, with IC50s of 26 and 18 nM for human COX-2 in human osteosarcoma cells and Chinese hamster ovary cells, with a 1000-fold selectivity for COX-2 over human COX-1 (IC50 > 50 μM in U937 cells and > 15 μM in Chinese hamster ovary cells). |
|---|---|
| Related Catalog | |
| Target |
Human COX-2:18 nM (IC50, in Chinese hamster ovary cells) Human COX-2:26 nM (IC50, in human osteosarcoma cells) |
| In Vitro | Rofecoxib (MK-0966) is a potent and orally active inhibitor of COX-2, with IC50s of 26 and 18 nM for human COX-2 in human osteosarcoma cells and Chinese hamster ovary cells, with a 1000-fold selectivity for COX-2 over COX-1 (IC50 >50 μM in U937 cells and >15 μM in Chinese hamster ovary cells). Rofecoxib time dependently inhibits purified human recombinant COX-2 (IC50=0.34 μM) but suppresses purified human COX-1 in a non-time-dependent manner that can only be observed at a very low substrate concentration (IC50=26 μM at 0.1 μM arachidonic acid concentration). Rofecoxib selectively inhibits lipopolysaccharide-induced, COX-2-derived PGE(2) synthesis with an IC50 value of 0.53 ± 0.02 μM compared with an IC50 value of 18.8 ± 0.9 μM for the inhibition of COX-1-derived thromboxane B(2) synthesis after blood coagulation[1]. Rofecoxib (36 μM) causes a cell proliferation of 68% in MPP89, of 58% in Ist-Mes-1 and 40% in Ist-Mes-2. MSTO-211H and NCI-H2452 treated with 36 μM of Rofecoxib have a survival of 97% and 90% respectively. Rofecoxib (36 μM) decreases COX-2 and mRNA levels in Ist-Mes-1, Ist-Mes-2 and MPP89 cell lines[3]. |
| In Vivo | Rofecoxib potently inhibits carrageenan-induced paw edema (ID50=1.5 mg/kg), carrageenan-induced paw hyperalgesia (ID50=1.0 mg/kg), lipopolysaccharide-induced pyresis (ID50=0.24 mg/kg), and adjuvant-induced arthritis (ID50=0.74 mg/kg/day) in rodent models. Rofecoxib also protects adjuvant-induced destruction of cartilage and bone structures in rats. In a 51Cr excretion assay for detection of gastrointestinal integrity in either rats or squirrel monkeys, rofecoxib shows no effect at doses up to 200 mg/kg/day for 5 days[1]. Rofecoxib (15 mg/kg, i.p.) reduces the blood vessels attached to the internal limiting membrane (ILM) in mice. COX-2 and VEGF protein expressions, COX-2 mRNA and VEGF mRNA are also significantly decreased by Rofecoxib in ROP mice[2]. |
| Cell Assay | The anti-proliferative activity of single drug treatments is assessed in a monolayer culture condition by plating Ist-Mes-1, Ist-Mes-2 and MPP89 cells in T25 flask. After 24 h, DMSO (at the same final concentration of that present in medium with drugs), 50 μM gefitinib or 36 μM Rofecoxib are added. The cells are then harvested at 48 h after treatment and analyzed by western blot and RT-PCR to evaluate the effect of the drugs on expression and mRNA levels of EGFR and COX-2. The expression of the cell cycle arrest genes and p-AKT, AKT, p-ERK and ERK is detected by Western blot to assess the antiproliferative activity of the two drugs in isolation (25 μM gefitinib or 4 μM Rofecoxib) and in combination 25 μM gefitinib+4 μM Rofecoxib[3]. |
| Animal Admin | Mice[2] Retinopathy of prematurity (ROP) is induced in C57BL/6J mice. The mice are randomly allocated into three experimental groups with 16 mice in each group: normal group-age-matched mice are maintained in room air from birth to P17 and are served as negative control; untreated ROP group-ROP is induced as described above without treatment and served as positive control; Rofecoxib-treated ROP group-ROP mice are treated daily with Rofecoxib (15 mg/kg body weight, intraperitoneally) from P12 to P17. Rofecoxib is dissolved in a 0.5% aqueous methylcellulose solution before administration[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 577.6±50.0 °C at 760 mmHg |
| Melting Point | 207°C |
| Molecular Formula | C17H14O4S |
| Molecular Weight | 314.356 |
| Flash Point | 303.1±30.1 °C |
| Exact Mass | 314.061279 |
| PSA | 68.82000 |
| LogP | 1.34 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.619 |
| Storage condition | Refrigerator |
| Precursor 10 | |
|---|---|
| DownStream 2 | |
| HS Code | 2932209090 |
|---|---|
| Summary | 2932209090. other lactones. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Validation of cyclooxygenase-2 as a direct anti-inflammatory target of 4-O-methylhonokiol in zymosan-induced animal models.
Arch. Pharm. Res. 38 , 813-25, (2015) 4-O-methylhonokiol (MH) is known to inhibit inflammation by partially understood mechanisms. Here, the anti-inflammatory mechanisms of MH were examined using enzymatic, cellular, and animal assays. In... |
|
|
Celecoxib increases lung cancer cell lysis by lymphokine-activated killer cells via upregulation of ICAM-1.
Oncotarget 6 , 39342-56, (2015) The antitumorigenic mechanism of the selective cyclooxygenase-2 (COX-2) inhibitor celecoxib is still a matter of debate. Using lung cancer cell lines (A549, H460) and metastatic cells derived from a l... |
|
|
A human ether-á-go-go-related (hERG) ion channel atomistic model generated by long supercomputer molecular dynamics simulations and its use in predicting drug cardiotoxicity
Toxicol. Lett. 230(3) , 382-92, (2014) • Current hERG assays remain insensitive as predictors of clinical toxicity. • We have developed a state-of-art computational model of hERG blocking. • The method was validated using two biological as... |
| Rofecoxid |
| 4-(p-(Methylsulfonyl)phenyl)-3-phenyl-2(5H)-furanone |
| Rofecoxib |
| 2(5H)-Furanone, 4-[4-(methyl-sulfonyl)phenyl]-3-phenyl- |
| 4-[4-(MethylSLdfonyl)phenyl]-3-phenyl-2(5H)-furanone |
| 4-[4-(methylsulfonyl)phenyl]-3-phenylfuran-2(5H)-one |
| 4-[4-(Methylsulfonyl)phenyl]-3-phenyl-2(5H)-furanone |
| 3-[phenyl-4-(4-methylsulphonyl)phenyl]-2-(5H)-furanone |
| 2(5H)-Furanone, 4-(4-(methylsulfonyl)phenyl)-3-phenyl- |
| MFCD00935806 |
| Vioxx |
| Ceoxx |
| MK-0966 |
| 4-[4-(Methylsulfonyl)phenyl]-3-phenyl |
| 4-(4-(methylsulfonyl)phenyl)-3-phenylfuran-2(5H)-one |
| 2(5H)-Furanone, 4-[4-(methylsulfonyl)phenyl]-3-phenyl- |
| 2(5H)-Furanone,4-4-(methylsulfonyl)phenyl-3-phenyl |
| 4-(4-methylsulfonylphenyl)-3-phenyl-2,5-dihydrofuran-2-one |
| Ceeoxx |
| 4-(4-methanesulfonylphenyl)-3-phenyl-5H-furan-2-one |
 CAS#:103-82-2
CAS#:103-82-2![2-Bromo-1-[4-(methylsulfonyl)phenyl]ethanone Structure](https://image.chemsrc.com/caspic/253/50413-24-6.png) CAS#:50413-24-6
CAS#:50413-24-6 CAS#:162012-30-8
CAS#:162012-30-8![[2-(4-methylsulfonylphenyl)-2-oxoethyl] 2-phenylacetate Structure](https://image.chemsrc.com/caspic/289/201737-94-2.png) CAS#:201737-94-2
CAS#:201737-94-2 CAS#:105-36-2
CAS#:105-36-2 CAS#:487047-31-4
CAS#:487047-31-4 CAS#:98-80-6
CAS#:98-80-6 CAS#:7732-18-5
CAS#:7732-18-5 CAS#:121-44-8
CAS#:121-44-8 CAS#:38654-91-0
CAS#:38654-91-0 CAS#:185147-17-5
CAS#:185147-17-5 CAS#:179174-76-6
CAS#:179174-76-6