Carbetocin acetate
Modify Date: 2024-01-09 22:28:02
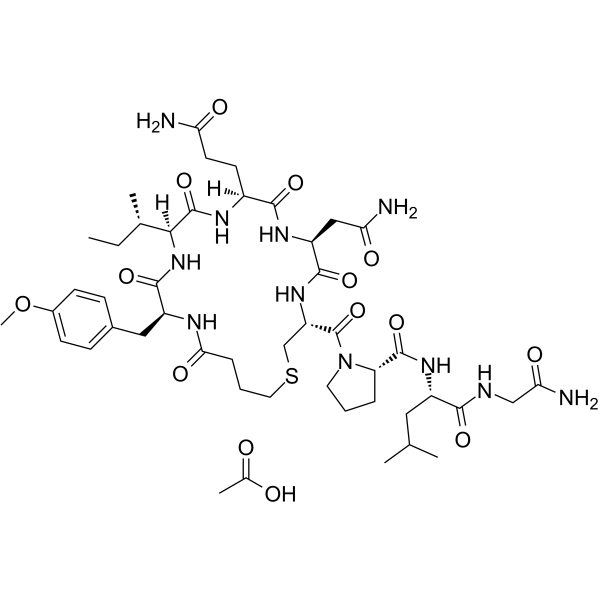
Carbetocin acetate structure
|
Common Name | Carbetocin acetate | ||
|---|---|---|---|---|
| CAS Number | 1631754-28-3 | Molecular Weight | 1048.21 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C47H73N11O14S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Carbetocin acetateCarbetocin acetate, an oxytocin (OT) analogue, is an oxytocin receptor agonist with a Ki of 7.1 nM. Carbetocin acetate has high affinity to chimeric N-terminus (E1) of the oxytocin receptor (Ki=1.17 μM). Carbetocin acetate has the potential for postpartum hemorrhage research. Carbetocin acetate can crosse the blood-brain barrier and produces antidepressant-like activity via activation of oxytocin receptors in the CNS[1][2][3]. |
| Name | 1-{[(3R,6S,9S,12S,15S)-6-(2-Amino-2-oxoethyl)-9-(3-amino-3-oxopropyl)-12-[(2S)-2-butanyl]-15-(4-methoxybenzyl)-5,8,11,14,17-pentaoxo-1-thia-4,7,10,13,16-pentaazacycloicosan-3-yl]carbonyl}-L-prolyl-L-leucylglycinamide acetate (1:1) |
|---|---|
| Synonym | More Synonyms |
| Description | Carbetocin acetate, an oxytocin (OT) analogue, is an oxytocin receptor agonist with a Ki of 7.1 nM. Carbetocin acetate has high affinity to chimeric N-terminus (E1) of the oxytocin receptor (Ki=1.17 μM). Carbetocin acetate has the potential for postpartum hemorrhage research. Carbetocin acetate can crosse the blood-brain barrier and produces antidepressant-like activity via activation of oxytocin receptors in the CNS[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Carbetocin acetate is an agonist with about 10-fold lower affinity for the oxytocin receptor but with significantly higher stability and a longer duration of action. Carbetocin acetate has higher affinity to the chimeric E1 receptor and especially to each of the combinations of E1 with the other extracellular domains, i.e. chimeric receptors E13 (Ki=13 nM), E123 (Ki=56 nM), and E1234 (Ki=37 nM)[2]. |
| In Vivo | Carbetocin (2-20 mg/kg; i.p.) acetate has a significant effect of treatment on the percent time spent climbing, swimming and immobile[1]. Carbetocin (1, 10,100 μg/rat, i.c.v.) acetate reveals a dose-dependent increase in the percent time spent swimming and a corresponding reduction in immobility following acute administration of 100 μg/rat[1]. Animal Model: Male Sprague-Dawley rats weighing between 300 and 500 g[1] Dosage: 2, 6.4, 20 mg/kg Administration: IP; single dose Result: Increased climbing with 6.4 mg/kg and resulted in a significantly greater proportion of time spent swimming with 20 mg/kg. |
| References |
| Molecular Formula | C47H73N11O14S |
|---|---|
| Molecular Weight | 1048.21 |
| Exact Mass | 1047.505859 |
| 1-{[(3R,6S,9S,12S,15S)-6-(2-Amino-2-oxoethyl)-9-(3-amino-3-oxopropyl)-12-[(2S)-2-butanyl]-15-(4-methoxybenzyl)-5,8,11,14,17-pentaoxo-1-thia-4,7,10,13,16-pentaazacycloicosan-3-yl]carbonyl}-L-prolyl-L-leucylglycinamide acetate (1:1) |
| Glycinamide, 1-[[(3R,6S,9S,12S,15S)-6-(2-amino-2-oxoethyl)-9-(3-amino-3-oxopropyl)-15-[(4-methoxyphenyl)methyl]-12-[(1S)-1-methylpropyl]-5,8,11,14,17-pentaoxo-1-thia-4,7,10,13,16-pentaazacycloeicos-3-yl]carbonyl]-L-prolyl-L-leucyl-, acetate (1:1) |
| MFCD10566502 |