tolylboronic acid
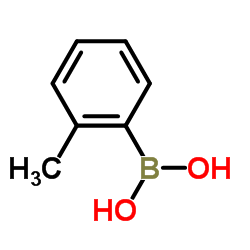
tolylboronic acid structure
|
Common Name | tolylboronic acid | ||
|---|---|---|---|---|
| CAS Number | 16419-60-6 | Molecular Weight | 135.96 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 283.4±33.0 °C at 760 mmHg | |
| Molecular Formula | C7H9BO2 | Melting Point | 162-164 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 125.2±25.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of tolylboronic acid2-Tolylboronic acid is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 2-Tolylboronic acid |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Tolylboronic acid is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 283.4±33.0 °C at 760 mmHg |
| Melting Point | 162-164 °C(lit.) |
| Molecular Formula | C7H9BO2 |
| Molecular Weight | 135.96 |
| Flash Point | 125.2±25.4 °C |
| Exact Mass | 136.069565 |
| PSA | 40.46000 |
| LogP | 2.05 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.528 |
| Storage condition | 0-6°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | ED7777777 |
| HS Code | 2931900090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2931900090 |
|---|---|
| Summary | 2931900090. other organo-inorganic compounds. VAT:17.0%. Tax rebate rate:13.0%. Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward). MFN tariff:6.5%. General tariff:30.0% |
|
Ruthenium(0)-catalyzed sp3 C-H bond arylation of benzylic amines using arylboronates.
Org. Lett. 7th ed., 14 , 1930-1933, (2012) A Ru-catalyzed direct arylation of benzylic sp(3) carbons of acyclic amines with arylboronates is reported. This highly regioselective and efficient transformation can be performed with various combin... |
|
|
Design and synthesis of active site inhibitors of the human farnesyl pyrophosphate synthase: apoptosis and inhibition of ERK phosphorylation in multiple myeloma cells.
J. Med. Chem. 7th ed., 55 , 3201-3215, (2012) Human farnesyl pyrophosphate synthase (hFPPS) controls intracellular levels of FPP and post-translational prenylation of small GTPase proteins, which are essential for cell signaling and cell prolifer... |
|
|
Efficient chiral monophosphorus ligands for asymmetric Suzuki-Miyaura coupling reactions.
Org. Lett. 9th ed., 14 , 2258-2261, (2012) A series of novel P-chiral monophosphorus ligands exhibit efficiency in asymmetric Suzuki-Miyaura coupling reactions, enabling the construction of an array of chiral biaryl products in high yields and... |
| 2-Methylbenzeneboronic acid |
| (2-Methylphenyl)boronic acid |
| 2-Tolylboronic acid |
| Boronic acid, B-(2-methylphenyl)- |
| 2-methylphenylboronic acid |
| MFCD00093526 |
| o-Tolylboronic acid |
| tolylboronic acid |
 CAS#:95-46-5
CAS#:95-46-5 CAS#:66107-34-4
CAS#:66107-34-4 CAS#:615-37-2
CAS#:615-37-2 CAS#:95-49-8
CAS#:95-49-8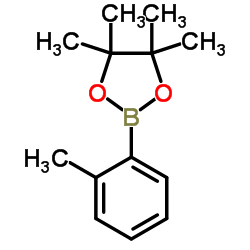 CAS#:195062-59-0
CAS#:195062-59-0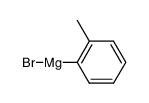 CAS#:932-31-0
CAS#:932-31-0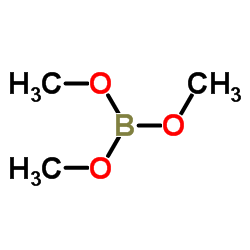 CAS#:121-43-7
CAS#:121-43-7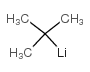 CAS#:594-19-4
CAS#:594-19-4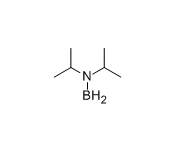 CAS#:22092-92-8
CAS#:22092-92-8 CAS#:7732-18-5
CAS#:7732-18-5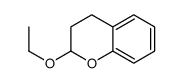 CAS#:10419-35-9
CAS#:10419-35-9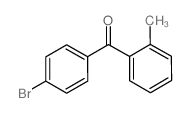 CAS#:27428-59-7
CAS#:27428-59-7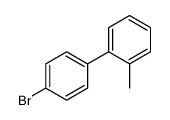 CAS#:106475-19-8
CAS#:106475-19-8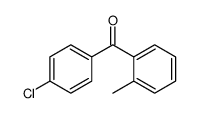 CAS#:41064-50-0
CAS#:41064-50-0![2'-METHYL-[1,1'-BIPHENYL]-4-CARBOXYLIC ACID structure](https://image.chemsrc.com/caspic/035/5748-43-6.png) CAS#:5748-43-6
CAS#:5748-43-6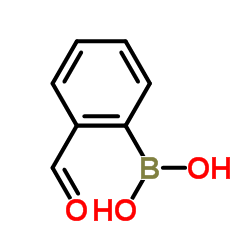 CAS#:40138-16-7
CAS#:40138-16-7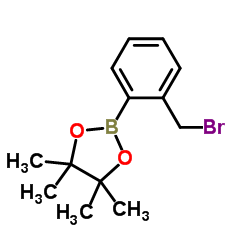 CAS#:377780-72-8
CAS#:377780-72-8 CAS#:40291-39-2
CAS#:40291-39-2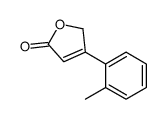 CAS#:143392-24-9
CAS#:143392-24-9 CAS#:94-69-9
CAS#:94-69-9
