naltrexone
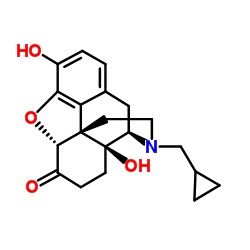
naltrexone structure
|
Common Name | naltrexone | ||
|---|---|---|---|---|
| CAS Number | 16590-41-3 | Molecular Weight | 341.40 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 558.1±50.0 °C at 760 mmHg | |
| Molecular Formula | C20H23NO4 | Melting Point | 168-170ºC | |
| MSDS | N/A | Flash Point | 291.4±30.1 °C | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of naltrexoneNaltrexone is an antagonist of Opioid receptor. Naltrexone inhibits cell proliferation in vivo. Naltrexone reduces tumor growth by interfering with cell signalling and modifying the immune system[1]. |
| Name | naltrexone |
|---|---|
| Synonym | More Synonyms |
| Description | Naltrexone is an antagonist of Opioid receptor. Naltrexone inhibits cell proliferation in vivo. Naltrexone reduces tumor growth by interfering with cell signalling and modifying the immune system[1]. |
|---|---|
| Related Catalog | |
| References |
[7]. Naltrexone |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 558.1±50.0 °C at 760 mmHg |
| Melting Point | 168-170ºC |
| Molecular Formula | C20H23NO4 |
| Molecular Weight | 341.40 |
| Flash Point | 291.4±30.1 °C |
| Exact Mass | 341.162720 |
| PSA | 70.00000 |
| LogP | 1.80 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.709 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Hazard Codes | F,T |
| Risk Phrases | 11-23/24/25-39/23/24/25 |
| Safety Phrases | 16-36/37-45 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| Precursor 9 | |
|---|---|
| DownStream 6 | |
|
Novel orally available salvinorin A analog PR-38 inhibits gastrointestinal motility and reduces abdominal pain in mouse models mimicking irritable bowel syndrome.
J. Pharmacol. Exp. Ther. 350(1) , 69-78, (2014) The opioid and cannabinoid systems play a crucial role in multiple physiological processes in the central nervous system and in the periphery. Selective opioid as well as cannabinoid (CB) receptor ago... |
|
|
Desensitization of functional µ-opioid receptors increases agonist off-rate.
Mol. Pharmacol. 86(1) , 52-61, (2014) Desensitization of µ-opioid receptors (MORs) develops over 5-15 minutes after the application of some, but not all, opioid agonists and lasts for tens of minutes after agonist removal. The decrease in... |
|
|
Treatment of alcohol dependence: recent progress and reduction of consumption.
Minerva Med. 105(6) , 447-66, (2014) Alcohol dependence (AD) is a major public health problem. Currently, three drugs for the treatment of AD have been approved by both the European Medicines Agency (EMA) and the Food and Drug Administra... |
| Morphinan-6-one, 17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxy-, (5α)- |
| Trexonil |
| NeMexin |
| (1S,5R,13R,17S)-4-(Cyclopropylmethyl)-10,17-dihydroxy-12-oxa-4-azapentacyclo[9.6.1.0.0.0]octadeca-7(18),8,10-trien-14-on |
| Depotrex |
| ReVia |
| en1939 |
| Naltrel |
| en1639 |
| Naltrexone |
| Vivitrol |
| (5α)-17-(Cyclopropylmethyl)-3,14-dihydroxy-4,5-epoxymorphinan-6-one |
| Antaxone |
| trexan |
| (1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydroxy-12-oxa-4-azapentacyclo[9.6.1.0.0.0]octadeca-7(18),8,10-trien-14-one |
| (1S,5R,13R,17S)-4-(cyclopropylméthyl)-10,17-dihydroxy-12-oxa-4-azapentacyclo[9.6.1.0.0.0]octadéca-7(18),8,10-trién-14-one |
| celupan |
| En 1639A |
| Um-792 |
| (5a)-17-(Cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxymorphinan-6-one |
| Nalorex |
| Depade |
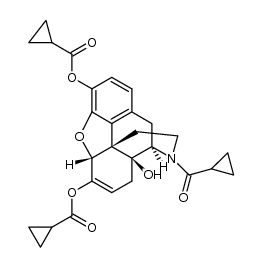 CAS#:1407490-23-6
CAS#:1407490-23-6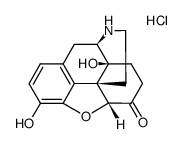 CAS#:52446-24-9
CAS#:52446-24-9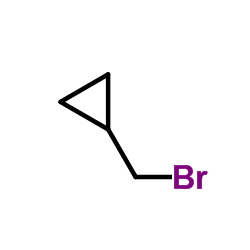 CAS#:7051-34-5
CAS#:7051-34-5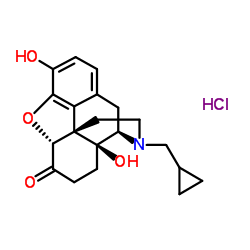 CAS#:16676-29-2
CAS#:16676-29-2 CAS#:33522-95-1
CAS#:33522-95-1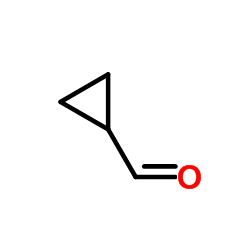 CAS#:1489-69-6
CAS#:1489-69-6 CAS#:2516-33-8
CAS#:2516-33-8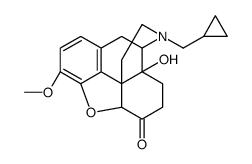 CAS#:16617-07-5
CAS#:16617-07-5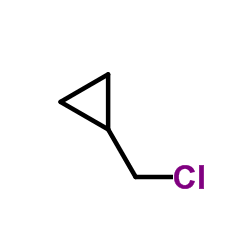 CAS#:5911-08-0
CAS#:5911-08-0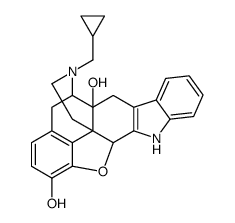 CAS#:111555-53-4
CAS#:111555-53-4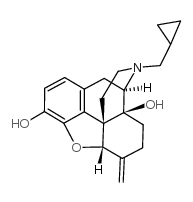 CAS#:55096-26-9
CAS#:55096-26-9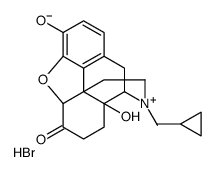 CAS#:73232-52-7
CAS#:73232-52-7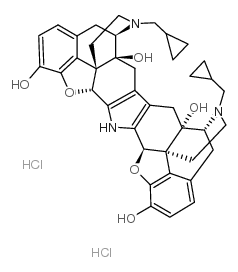 CAS#:105618-26-6
CAS#:105618-26-6 CAS#:161532-22-5
CAS#:161532-22-5