Furagin
Modify Date: 2024-01-09 01:27:12
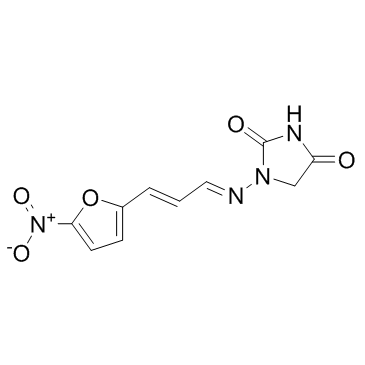
Furagin structure
|
Common Name | Furagin | ||
|---|---|---|---|---|
| CAS Number | 1672-88-4 | Molecular Weight | 264.194 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C10H8N4O5 | Melting Point | 267-270ºC (dec.) | |
| MSDS | N/A | Flash Point | N/A | |
Use of FuraginFuragin, nitrofurantoin analog, is an anti-bacterial agent. Furagin is 2-substituted 5-nitrofuran, chemically and structurally similar to well-known antibacterial compound nitrofurantoin.IC50 Value: Target: Antibacterialin vitro: The furagin concentrations in serum remain several hours above the MIC concentrations of many pathogenic bacteria. Despite the high concentrations in serum, the urine levels of furagin were generally lower than those of nitrofurantoin. The 24 hr recoveries in urine were 8--13% for furagin and about 36% for nitrofurantoin [1].in vivo: A time-independent increase in SCE frequency was found in lymphocytes of children treated with furagin. Total CA frequency did not differ significantly between groups of children with various duration of furagin treatment [2]. Women were randomised into two groups receiving either ciprofloxacin 250mg twice a day for 3 days (n=13) or furagin 100mg three times a day for 7 days (n=14). Median lengths of follow-up were 4 days and 5 days in the ciprofloxacin and furagin groups, respectively [3]. |
| Name | 1-[(E)-[(E)-3-(5-nitrofuran-2-yl)prop-2-enylidene]amino]imidazolidine-2,4-dione |
|---|---|
| Synonym | More Synonyms |
| Description | Furagin, nitrofurantoin analog, is an anti-bacterial agent. Furagin is 2-substituted 5-nitrofuran, chemically and structurally similar to well-known antibacterial compound nitrofurantoin.IC50 Value: Target: Antibacterialin vitro: The furagin concentrations in serum remain several hours above the MIC concentrations of many pathogenic bacteria. Despite the high concentrations in serum, the urine levels of furagin were generally lower than those of nitrofurantoin. The 24 hr recoveries in urine were 8--13% for furagin and about 36% for nitrofurantoin [1].in vivo: A time-independent increase in SCE frequency was found in lymphocytes of children treated with furagin. Total CA frequency did not differ significantly between groups of children with various duration of furagin treatment [2]. Women were randomised into two groups receiving either ciprofloxacin 250mg twice a day for 3 days (n=13) or furagin 100mg three times a day for 7 days (n=14). Median lengths of follow-up were 4 days and 5 days in the ciprofloxacin and furagin groups, respectively [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Melting Point | 267-270ºC (dec.) |
| Molecular Formula | C10H8N4O5 |
| Molecular Weight | 264.194 |
| Exact Mass | 264.049469 |
| PSA | 120.73000 |
| LogP | 0.81 |
| Index of Refraction | 1.689 |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| HS Code | 2934999090 |
|---|
|
~% 
Furagin CAS#:1672-88-4 |
| Literature: Yakugaku Zasshi, , vol. 75, p. 117,119, 120 Chem.Abstr., , p. 1782 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| 1-((3-(5-Nitro-2-furyl)allylidene)amino)-hydantoin |
| Furagin |
| MFCD00431319 |
| 1-{[(1E,2E)-3-(5-Nitro-2-furyl)prop-2-en-1-ylidene]amino}imidazolidine-2,4-dione |
| 1-{(E)-[(2E)-3-(5-Nitro-2-furyl)-2-propen-1-ylidene]amino}-2,4-imidazolidinedione |
| 1-[3-(5-Nitro-[2]furyl)-allylidenamino]-imidazolidin-2,4-dion |
| Furazidin |
| 2,4-Imidazolidinedione, 1-[[(1E,2E)-3-(5-nitro-2-furanyl)-2-propen-1-ylidene]amino]- |
| Akritoin |
| Furazidine |
| 1-[3-(5-nitro-[2]furyl)-allylidenamino]-imidazolidine-2,4-dione |
| NF 416 |
| 1-((3-(5-Nitrofuran-2-yl)allylidene)amino)imidazolidine-2,4-dione |