Cyclohexane-d12
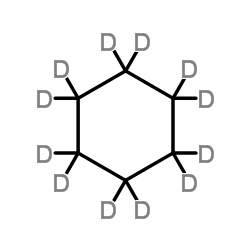
Cyclohexane-d12 structure
|
Common Name | Cyclohexane-d12 | ||
|---|---|---|---|---|
| CAS Number | 1735-17-7 | Molecular Weight | 96.233 | |
| Density | 0.8±0.1 g/cm3 | Boiling Point | 80.7±0.0 °C at 760 mmHg | |
| Molecular Formula | C6D12 | Melting Point | 6.5°C | |
| MSDS | Chinese USA | Flash Point | -18.3±0.0 °C | |
| Symbol |




GHS02, GHS07, GHS08, GHS09 |
Signal Word | Danger | |
| Name | cyclohexane-d12 |
|---|---|
| Synonym | More Synonyms |
| Density | 0.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 80.7±0.0 °C at 760 mmHg |
| Melting Point | 6.5°C |
| Molecular Formula | C6D12 |
| Molecular Weight | 96.233 |
| Flash Point | -18.3±0.0 °C |
| Exact Mass | 96.169220 |
| LogP | 3.39 |
| Vapour Pressure | 93.7±0.1 mmHg at 25°C |
| Index of Refraction | 1.433 |
| Storage condition | Flammables area |
| Stability | Stable. Highly flammable. Readily forms explosive mixtures with air. Note low flash point. Protect from moisture. Incompatible with strong oxidizing agents. |
Synonym:None Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: 11 36/37/38 Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Highly flammable. Irritating to eyes, respiratory system and skin.The toxicological properties of this material have not been fully investigated.Hygroscopic (absorbs moisture from the air).Highly flammable. Potential Health Effects Eye: May cause mild eye irritation. Causes eye irritation. Skin: Causes skin irritation. May be absorbed through the skin in harmful amounts. May cause irritation with burning pain, itching and redness. The toxicological properties of this material have not been fully investigated. Ingestion: Ingestion of large amounts may cause gastrointestinal irritation. Causes gastrointestinal irritation with nausea, vomiting and diarrhea. May cause liver and kidney damage. May cause central nervous system depression, characterized by excitement, followed by headache, dizziness, drowsiness, and nausea. Advanced stages may cause collapse, unconsciousness, coma and possible death due to respiratory failure. May cause vascular collapse and damage. The toxicological properties of this substance have not been fully investigated. Aspiration of material into the lungs may cause chemical pneumonitis, which may be fatal. Inhalation: Inhalation of high concentrations may cause central nervous system effects characterized by nausea, headache, dizziness, unconsciousness and coma. Causes respiratory tract irritation. May cause respiratory tract irritation. Irritation may lead to chemical pneumonitis and pulmonary edema. May be fatal if exposed to high concentrations. May cause pulmonary edema and severe respiratory disturbances. Vapors may cause dizziness or suffocation. Chronic: Prolonged or repeated skin contact may cause defatting and dermatitis. May cause liver and kidney damage. Section 4 - FIRST AID MEASURES Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Do NOT allow victim to rub eyes or keep eyes closed. Skin: Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists. Wash clothing before reuse. Ingestion: Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Possible aspiration hazard. Get medical aid immediately. Wash mouth out with water. Inhalation: Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask. Notes to Physician: Treat symptomatically and supportively. Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air. Vapors can travel to a source of ignition and flash back. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Extremely flammable. Material will readily ignite at room temperature. Use water spray to keep fire-exposed containers cool. Water may be ineffective. Material is lighter than water and a fire may be spread by the use of water. Liquid will float and may reignite on the surface of water. Flammable liquid and vapor. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. May polymerize explosively when involved in a fire. Will be easily ignited by heat, sparks or flame. Containers may explode when heated. Containers may explode if exposed to fire. Extinguishing Media: For small fires, use dry chemical, carbon dioxide, water spray or alcohol-resistant foam. Water may be ineffective. Water may spread fire. This material is lighter than water and insoluble in water. The fire could easily be spread by the use of water in an area where the water cannot be contained. If water is the only media available, use in flooding amounts. For large fires, use water spray, fog or alcohol-resistant foam. Do NOT use straight streams of water. For large fires, use water spray, fog or regular foam. For small fires, use dry chemical, carbon dioxide, water spray or regular foam. Cool containers with flooding quantities of water until well after fire is out. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Wear a self contained breathing apparatus and appropriate personal protection. (See Exposure Controls, Personal Protection section). Scoop up with a nonsparking tool, then place into a suitable container for disposal. Remove all sources of ignition. Provide ventilation. Section 7 - HANDLING and STORAGE Handling: Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use only in a well-ventilated area. Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Do not reuse this container. Avoid breathing dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame. Do not get on skin or in eyes. Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames. Storage: Keep away from heat and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Flammables-area. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low. Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits. Exposure Limits CAS# 1735-17-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear neoprene gloves, apron, and/or clothing. Wear nitrile-latex gloves, apron, and/or clothing. Use polyvinyl alcohol or fluorocarbon rubber (viton) gloves. Clothing: Wear nitrile-latex gloves, apron, and/or clothing. Wear appropriate protective clothing to prevent skin exposure. Respirators: Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Liquid Color: colorless Odor: sweetish odor - chloroform-like pH: Not available. Vapor Pressure: 95 mm Hg Viscosity: 1.02 cP at 63F. Boiling Point: 80.7 deg C Freezing/Melting Point: 6.5 deg C Autoignition Temperature: 245 deg C ( 473.00 deg F) Flash Point: -20 deg C ( -4.00 deg F) Explosion Limits, lower: 1.3 Explosion Limits, upper: 8.0 Decomposition Temperature: Not available. Solubility in water: Practically insoluble in water. Specific Gravity/Density: 0.8 (Water=1) Molecular Formula: CH2(CH2)4CH2 Molecular Weight: 84.084 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable at room temperature in closed containers under normal storage and handling conditions. Conditions to Avoid: Mechanical shock, incompatible materials, ignition sources, excess heat, exposure to moist air or water, oxidizers. Incompatibilities with Other Materials: Strong oxidizing agents, nitrogen dioxide. Hazardous Decomposition Products: Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide. Hazardous Polymerization: Has not been reported. Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 1735-17-7 unlisted. LD50/LC50: Not available. Carcinogenicity: Cyclohexane-D12 - Not listed by ACGIH, IARC, or NTP. Section 12 - ECOLOGICAL INFORMATION Ecotoxicity: Fish: Fathead Minnow: LC50 = 117.0 mg/L; 96 Hr.; Static conditionsFish: Bluegill/Sunfish: LC50 = 34.72 mg/L; 96 Hr.; 25 degrees CWater flea Daphnia: EC50 = 400.00 mg/L; 48 Hr.; UnspecifiedBacteria: Phytobacterium phosphoreum: EC50 = 227.00 mg/L; 5, 30 minutes; Microtox test Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. Section 14 - TRANSPORT INFORMATION IATA Shipping Name: CYCLOHEXANE Hazard Class: 3 UN Number: 1145 Packing Group: II IMO Shipping Name: CYCLOHEXANE Hazard Class: 3.1 UN Number: 1145 Packing Group: II RID/ADR Shipping Name: CYCLOHEXANE Hazard Class: 3 UN Number: 1145 Packing group: II Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: XI F Risk Phrases: R 11 Highly flammable. R 36/37/38 Irritating to eyes, respiratory system and skin. Safety Phrases: S 9 Keep container in a well-ventilated place. S 16 Keep away from sources of ignition - No smoking. S 28A After contact with skin, wash immediately with plenty of water. S 33 Take precautionary measures against static discharges. S 37 Wear suitable gloves. S 45 In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible). WGK (Water Danger/Protection) CAS# 1735-17-7: No information available. Canada None of the chemicals in this product are listed on the DSL/NDSL list. CAS# 1735-17-7 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 1735-17-7 is not listed on the TSCA inventory. It is for research and development use only. SECTION 16 - ADDITIONAL INFORMATION N/A |
| Symbol |




GHS02, GHS07, GHS08, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H304-H315-H336-H410 |
| Precautionary Statements | P210-P261-P273-P301 + P310-P331-P501 |
| Hazard Codes | F:Highlyflammable;Xn:Harmful;N:Dangerousfortheenvironment; |
| Risk Phrases | R11;R38;R50/53;R65 |
| Safety Phrases | S9-S16-S25-S33-S60-S61-S62 |
| RIDADR | UN 1145 3/PG 2 |
| WGK Germany | 3 |
| Packaging Group | II |
| Hazard Class | 3 |
|
~% 
Cyclohexane-d12 CAS#:1735-17-7 |
| Literature: Journal of Chemical Physics, , vol. 8, p. 403,404 |
|
~% 
Cyclohexane-d12 CAS#:1735-17-7 |
| Literature: Advanced Synthesis and Catalysis, , vol. 351, # 4 p. 563 - 568 |

Cyclohexane-d12 CAS#:1735-17-7 ~% 
Cyclohexane-d12 CAS#:1735-17-7 |
| Literature: Journal of the American Chemical Society, , vol. 103, # 18 p. 5342 - 5348 |
| Precursor 3 | |
|---|---|
| DownStream 10 | |
|
A vibrational study of cyclohexane and some of its isotopic derivatives-I: Raman and infrared spectra and assignments of cyclohexane and cyclohexane-d12. Wiberg KB and Shrake A.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 27(7) , 1139-51, (1971)
|
|
|
Viscosities of benzene-d6 and cyclohexane-d12. Dixon JA and Schiessler RW.
J. Phys. Chem. 58(5) , 430-32, (1954)
|
|
|
Dynamic deuterium NMR in liquid crystalline solvents: Ring inversion of cyclohexane-d12. Poupko R and Luz Z.
J. Chem. Phys. 75(4) , 1675-1681, (1981)
|
| EINECS 217-077-3 |
| MFCD00044212 |
| Cyclohexane-d12 |
| (2H12)Cyclohexane |
| deuterated cyclohexane |
| Cyclohexane-d (Isotopic) |
| 1,1,2,2,3,3,4,4,5,5,6,6-dodecadeuteriocyclohexane |
| (H)Cyclohexane |
| Cyclohexane, d12 |
| Cyclohexane-d |

 CAS#:51209-49-5
CAS#:51209-49-5 CAS#:108-94-1
CAS#:108-94-1 CAS#:865-49-6
CAS#:865-49-6 CAS#:66522-78-9
CAS#:66522-78-9 CAS#:7698-05-7
CAS#:7698-05-7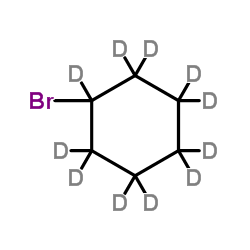 CAS#:35558-49-7
CAS#:35558-49-7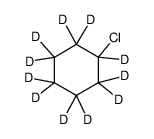 CAS#:119206-70-1
CAS#:119206-70-1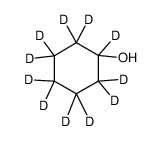 CAS#:93131-17-0
CAS#:93131-17-0