Daclatasvir-d6
Modify Date: 2023-01-17 13:12:14
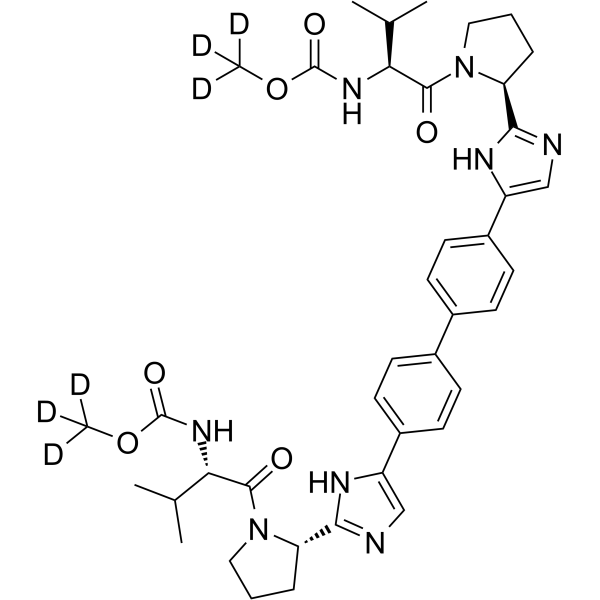
Daclatasvir-d6 structure
|
Common Name | Daclatasvir-d6 | ||
|---|---|---|---|---|
| CAS Number | 1801709-41-0 | Molecular Weight | 744.91 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 1071.2±65.0 °C at 760 mmHg | |
| Molecular Formula | C40H44D6N8O6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 601.7±34.3 °C | |
Use of Daclatasvir-d6Daclatasvir-d6 is deuterium labeled Daclatasvir. Daclatasvir (BMS-790052) is a potent and orally active HCV NS5A protein inhibitor with EC50s range of 9-146 pM for multiple HCV replicon genotypes. Daclatasvir is also a organic anion transporting polypeptide 1B (OATP1B) and OATP1B3 inhibitor with IC50s of 1.5 µM and 3.27 µM, respectively[1][2][3]. |
| Name | Daclatasvir-d6 |
|---|---|
| Synonym | More Synonyms |
| Description | Daclatasvir-d6 is deuterium labeled Daclatasvir. Daclatasvir (BMS-790052) is a potent and orally active HCV NS5A protein inhibitor with EC50s range of 9-146 pM for multiple HCV replicon genotypes. Daclatasvir is also a organic anion transporting polypeptide 1B (OATP1B) and OATP1B3 inhibitor with IC50s of 1.5 µM and 3.27 µM, respectively[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 1071.2±65.0 °C at 760 mmHg |
| Molecular Formula | C40H44D6N8O6 |
| Molecular Weight | 744.91 |
| Flash Point | 601.7±34.3 °C |
| Exact Mass | 744.422974 |
| LogP | 5.44 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.595 |
| Carbamic acid, N,N'-[[1,1'-biphenyl]-4,4'-diylbis[1H-imidazole-5,2-diyl(2S)-2,1-pyrrolidinediyl[(1S)-1-(1-methylethyl)-2-oxo-2,1-ethanediyl]]]bis-, dimethyl-d3 ester |
| Daclatasvir-d6 |
| (2H3)Methyl {(2S)-3-methyl-1-[(2S)-2-{5-[4'-(2-{(2S)-1-[(2S)-3-methyl-2-({[(2H3)methyloxy]carbonyl}amino)butanoyl]-2-pyrrolidinyl}-1H-imidazol-5-yl)-4-biphenylyl]-1H-imidazol-2-yl}-1-pyrrolidi
 nyl]-1-oxo-2-butanyl}carbamate |

